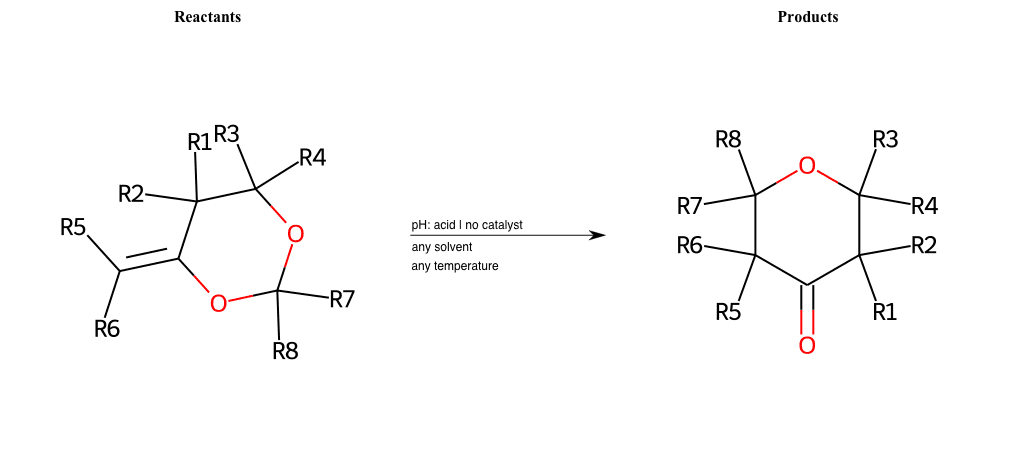
Pericyclic
# Petasis-Ferrier-Rearrangement
References:
[0]
Petasis-Ferrier Rearrangement
Special Conditions : SIDE REACTION Condition to enforce:
R1 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R2 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R4 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R5 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R7 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R8 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
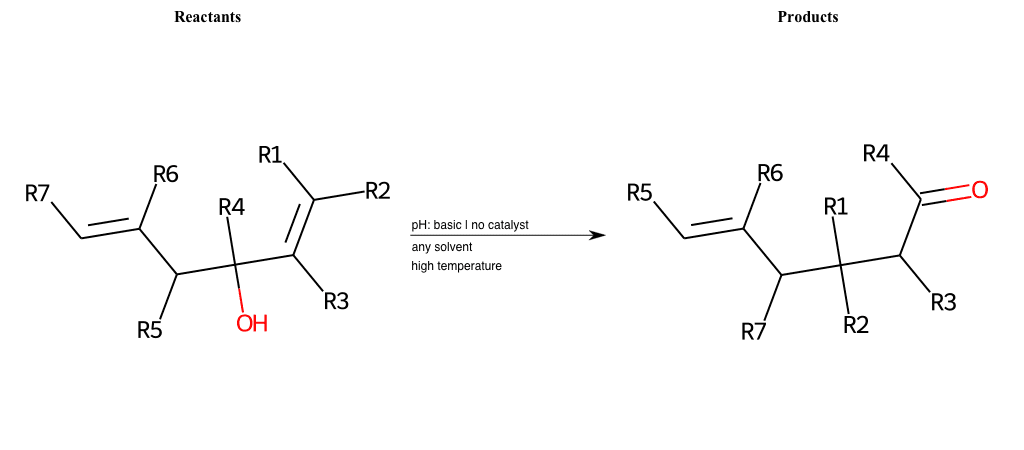
# Oxy-Cope-Rearrangement
References:
[0]
Cope rearrangement - Wikipedia
Special Conditions : SIDE REACTION Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = L-A
R7 = L-A
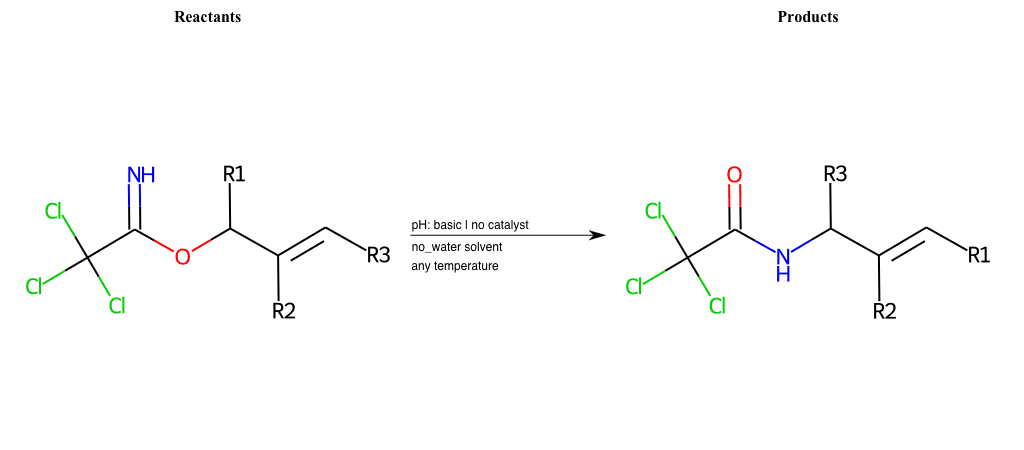
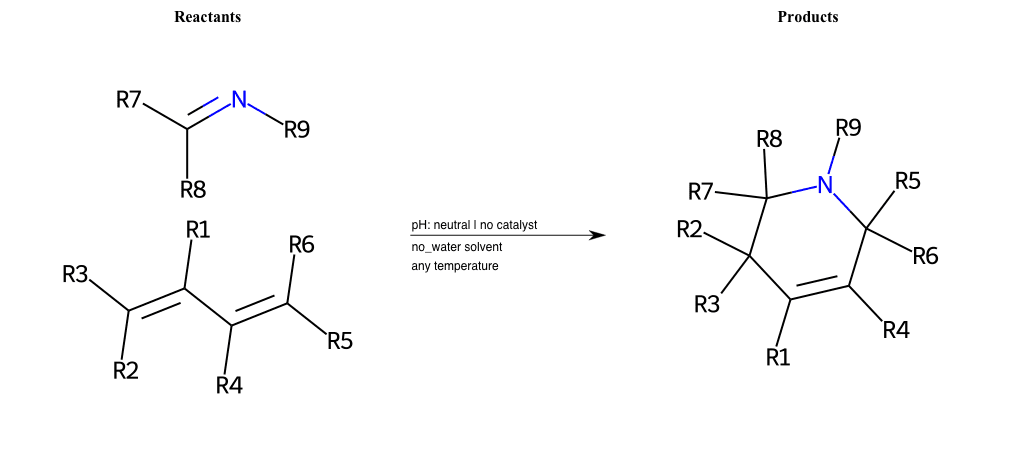
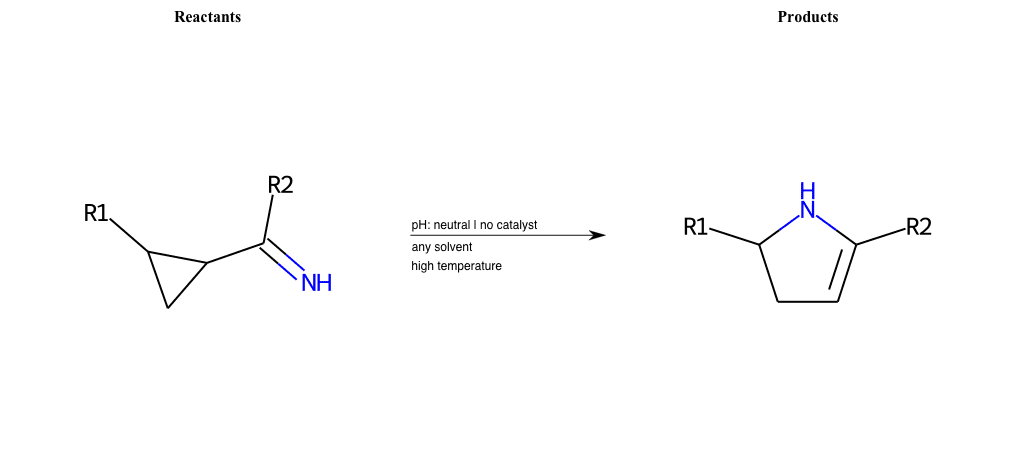
# Neber-Rearrangement
References:
[0]
Neber Rearrangement
Special Conditions : SIDE REACTION Condition to enforce:
R1 = L-A
R2 = L-A, Vinyl-Group
R3 = L-A, Vinyl-Group
R4 = L-A
# Claisen-Rearrangement
References:
[0]
Claisen rearrangement - Wikipedia
[1]
Claisen Rearrangement
Special Conditions : SIDE REACTION
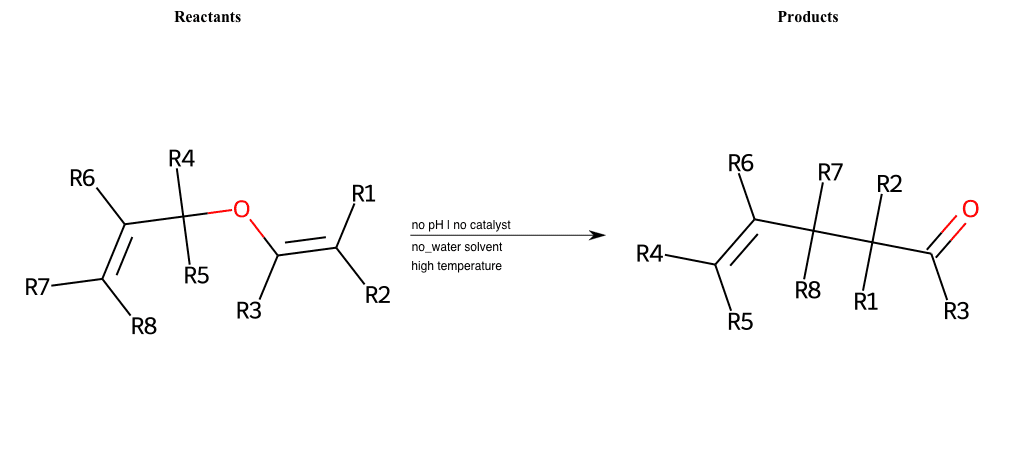
# Oxa-Vinylcyclopropane-Rearrangement
References:
[0]
Vinylcyclopropane rearrangement - Wikipedia
Special Conditions : SIDE REACTION Condition to enforce:
R1 = L-A, A-Aromatic-Carbon
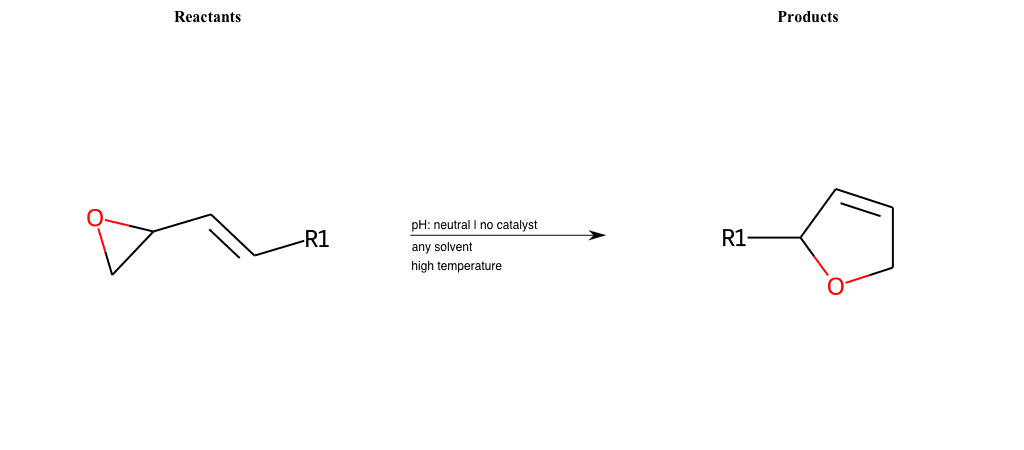
# Claisen-Rearrangement-Aromatic
References:
[0]
Claisen rearrangement - Wikipedia
[1]
Claisen Rearrangement
Special Conditions : SIDE REACTION
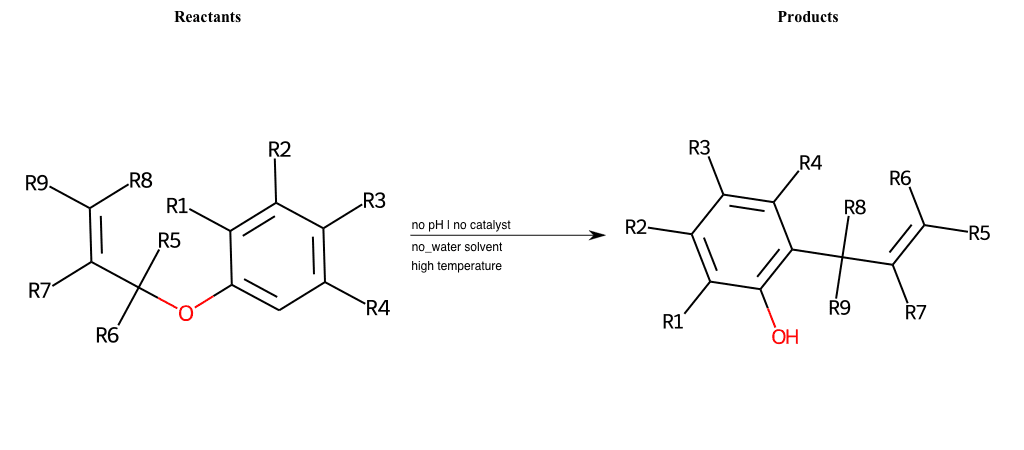
# Buchner-Ring-Expansion-C
References:
[0]
Pericyclic reaction - Wikipedia
Special Conditions : SIDE REACTION
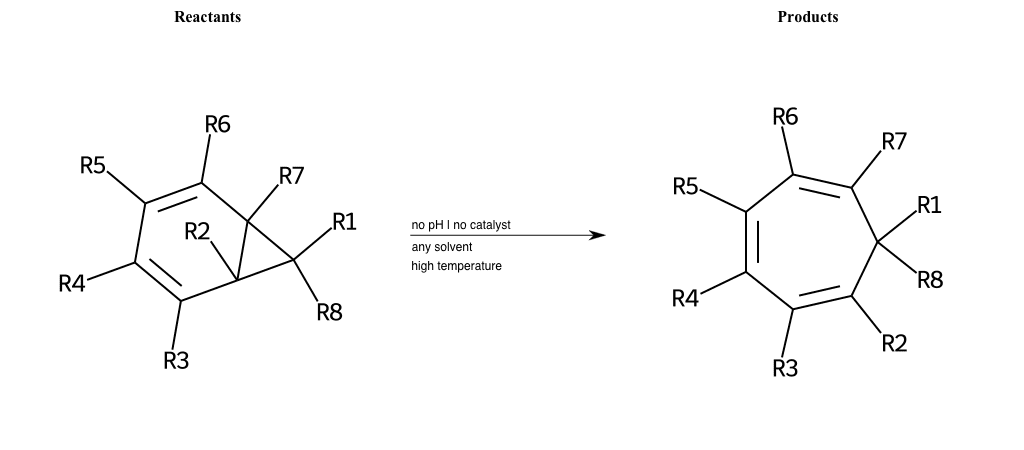
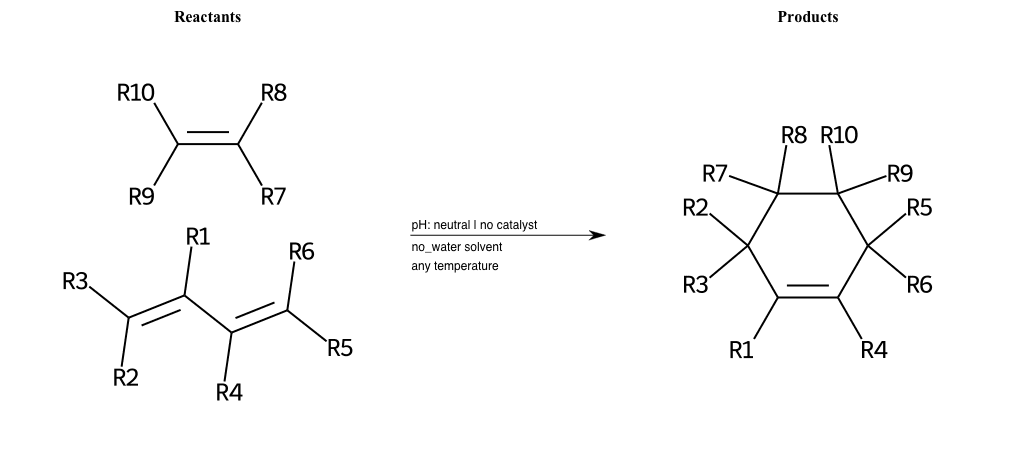
# Diels-Alder-4+2-Nitrogen
References:
[0]
diels-alder-reaction
[1]
Pi Donors And Resonance - Pi Donors Make Carbons More Nucleophilic
Condition to enforce:
R1 = H, A-Aliphatic-Carbon
R2 = L-A
R3 = L-A
R4 = H, A-Aliphatic-Carbon
R5 = L-A
R6 = L-A
R7 = L-A
R8 = L-A
R9 = L-A
# 1,3-Dipolar-Cycloaddition-Type-I
References:
[0]
1,3-Dipolar cycloaddition - Wikipedia

# Overman-Rearrangement-Pt1
References:
[0]
Overman Rearrangement
[1]
Overman Rearrangement
Condition to enforce:
R1 = H, A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon
# Ene-Reaction-C=C
References:
[0]
Ene reaction - Wikipedia
[1]
Ene Reaction
Condition to enforce:
R1 = L-A, A-Aromatic-Carbon
R2 = L-A, A-Aromatic-Carbon
R3 = L-A, A-Aromatic-Carbon
R4 = L-A, A-Aromatic-Carbon
R5 = L-A, A-Aromatic-Carbon
R6 = L-A, A-Aromatic-Carbon
R7 = L-A, A-Aromatic-Carbon
R8 = L-A, A-Aromatic-Carbon
R9 = L-A, A-Aromatic-Carbon

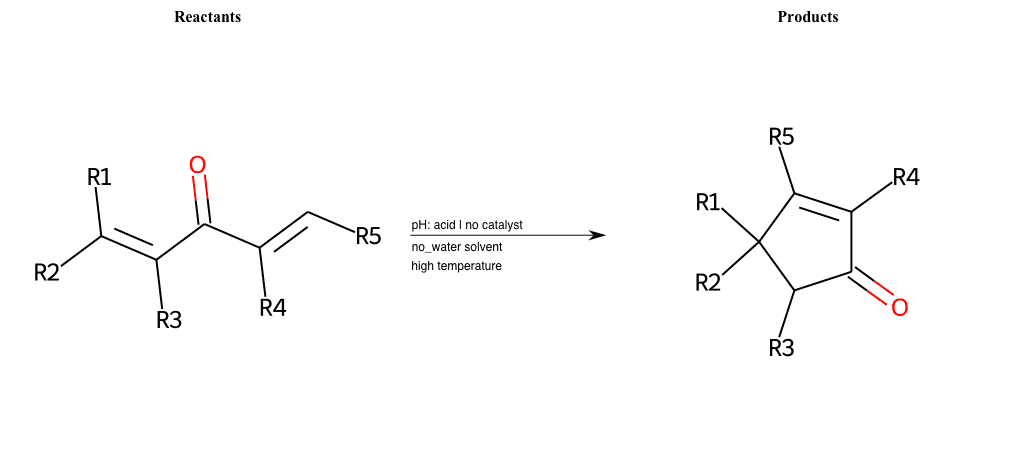
# Nazarov-Cyclization
References:
[0]
Nazarov Cyclization Reaction
Condition to enforce:
R1 = L-A, A-Aromatic-Carbon
R2 = L-A, A-Aromatic-Carbon
R3 = L-A, A-Aromatic-Carbon
R4 = L-A, A-Aromatic-Carbon
R5 = L-A, A-Aromatic-Carbon
# Diels-Alder-4+2
References:
[0]
diels-alder-reaction
[1]
Pi Donors And Resonance - Pi Donors Make Carbons More Nucleophilic
Condition to enforce:
R1 = H, A-Aliphatic-Carbon
R2 = L-A
R3 = L-A
R4 = H, A-Aliphatic-Carbon
R5 = L-A
R6 = L-A
R7 = L-A
R8 = L-A
R9 = L-A
R10 = L-A
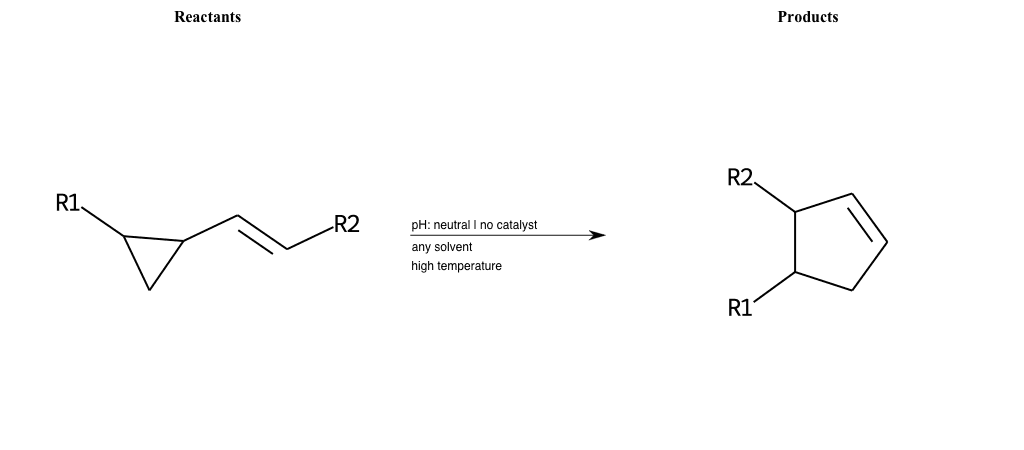
# Vinylcyclopropane-Rearrangement
References:
[0]
Vinylcyclopropane rearrangement - Wikipedia
Special Conditions : SIDE REACTION Condition to enforce:
R1 = L-A, A-Aromatic-Carbon
R2 = L-A, A-Aromatic-Carbon
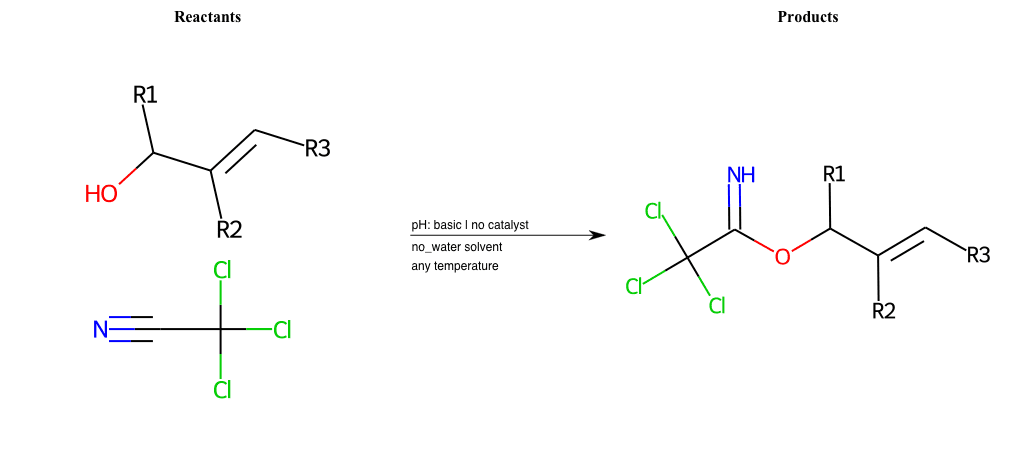
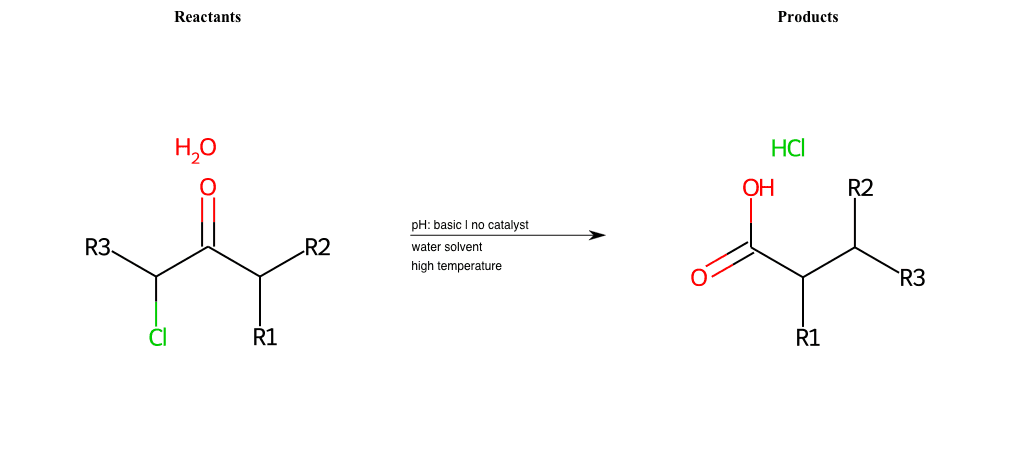
# Favorskii-Rearrangement-Cl
References:
[0]
Favorskii rearrangement - Wikipedia
[1]
favorskii-rearrangement-1.html
Condition to enforce:
R1 = L-A, A-Aromatic-Carbon
R2 = L-A, A-Aromatic-Carbon
R3 = L-A, A-Aromatic-Carbon
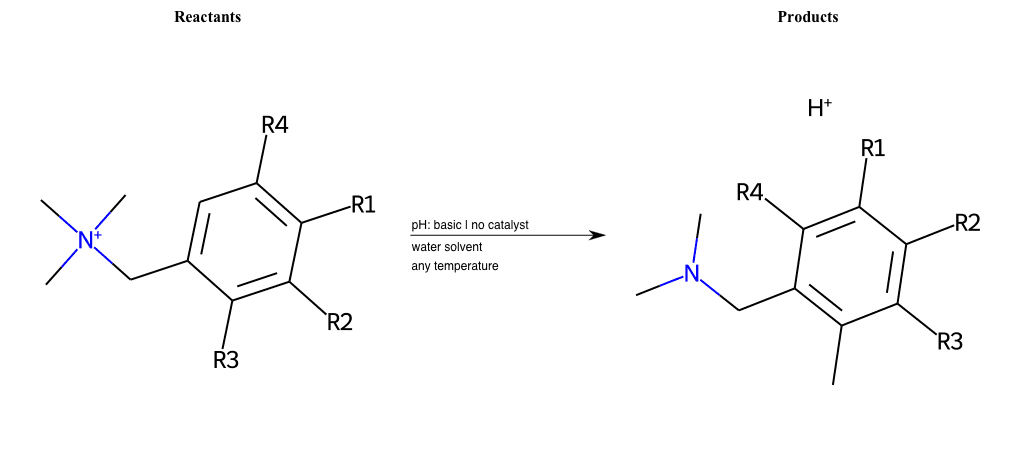
# Sommelet-Hauser-Rearrangement
References:
[0]
Sommelet–Hauser rearrangement - Wikipedia
Special Conditions : SIDE REACTION Condition to enforce:
R1 = L-A, A-Aromatic-Carbon
R2 = L-A, A-Aromatic-Carbon
R3 = L-A, A-Aromatic-Carbon
R4 = L-A, A-Aromatic-Carbon
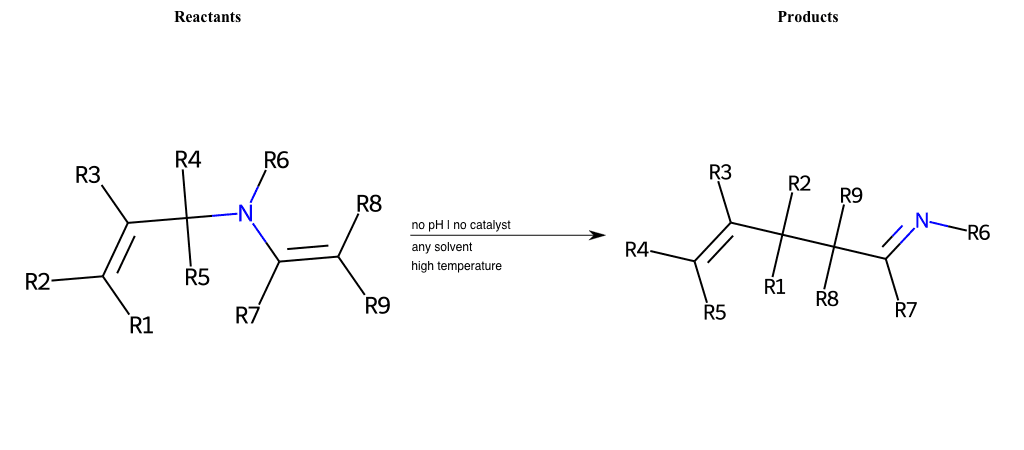
# Irreversable-Azo-Cope-Rearrangement
References:
[0]
Aza-Cope rearrangement - Wikipedia
Special Conditions : SIDE REACTION Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = L-A
R7 = L-A
R8 = L-A
R9 = L-A
# Aza-Vinylcyclopropane-Rearrangement
References:
[0]
Vinylcyclopropane rearrangement - Wikipedia
Special Conditions : SIDE REACTION Condition to enforce:
R1 = L-A, A-Aromatic-Carbon
R2 = L-A, A-Aromatic-Carbon
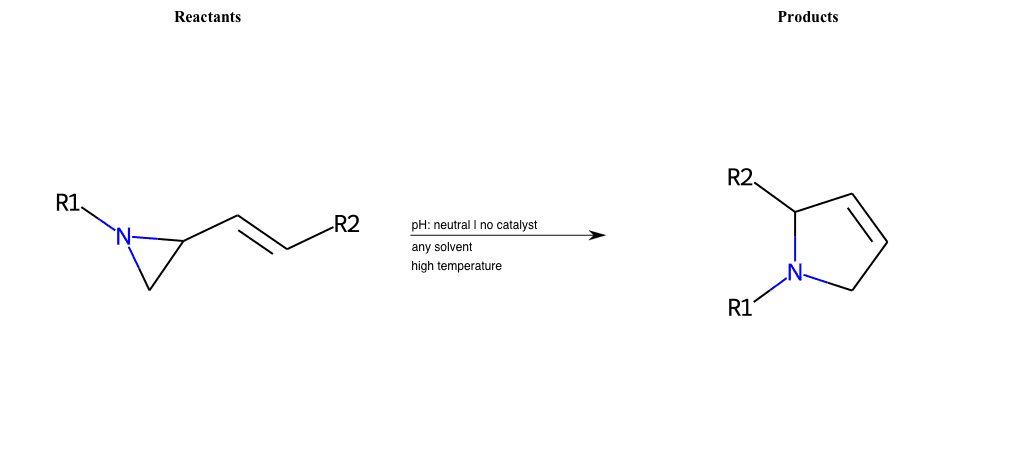
# Ene-Reaction-C=N
References:
[0]
Ene reaction - Wikipedia
[1]
Ene Reaction
Condition to enforce:
R1 = L-A, A-Aromatic-Carbon
R2 = L-A, A-Aromatic-Carbon
R3 = L-A, A-Aromatic-Carbon
R4 = L-A, A-Aromatic-Carbon
R5 = L-A, A-Aromatic-Carbon
R6 = L-A, A-Aromatic-Carbon
R7 = L-A, A-Aromatic-Carbon
R8 = L-A, A-Aromatic-Carbon

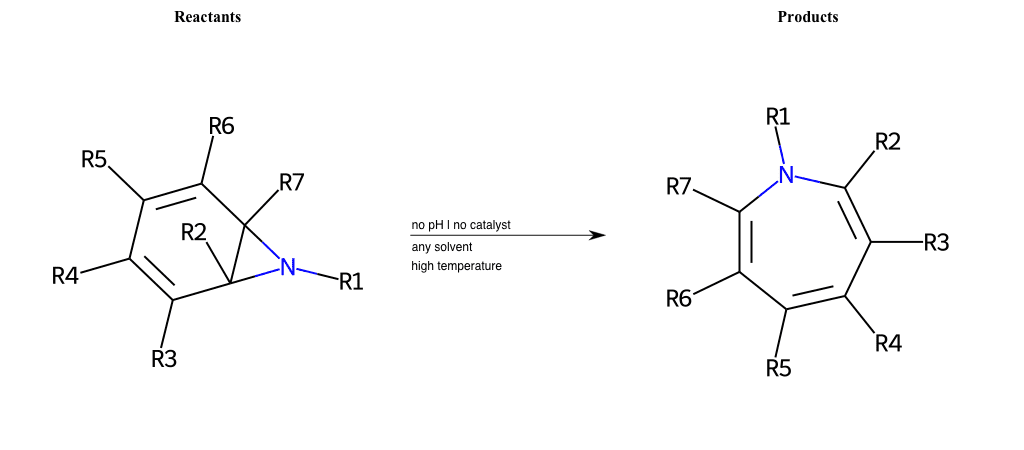
# Buchner-Ring-Expansion-N
References:
[0]
Pericyclic reaction - Wikipedia
Special Conditions : SIDE REACTION
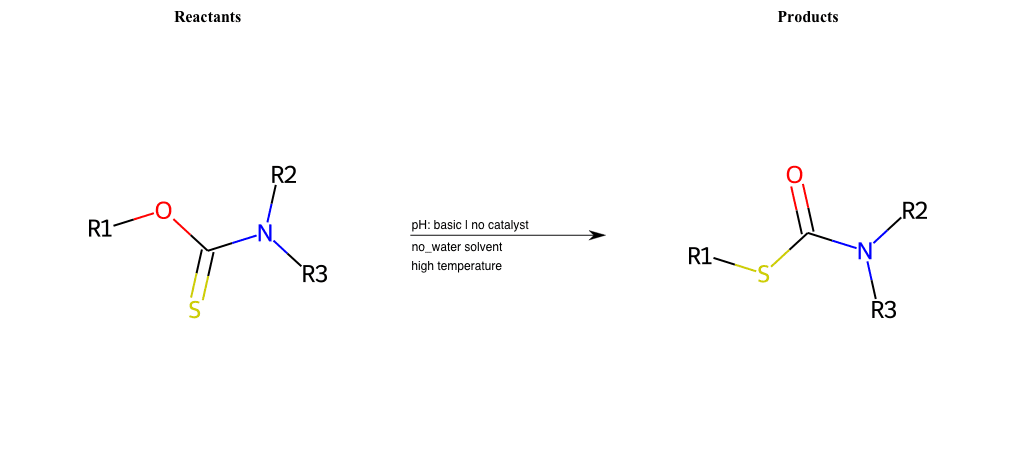
# Newman-Kwart-Rearrangement
References:
[0]
Newman-Kwart Rearrangement
Special Conditions : SIDE REACTION Condition to enforce:
R1 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R2 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
# Ene-Reaction-C=O
References:
[0]
Ene reaction - Wikipedia
[1]
Ene Reaction
Condition to enforce:
R1 = L-A, A-Aromatic-Carbon
R2 = L-A, A-Aromatic-Carbon
R3 = L-A, A-Aromatic-Carbon
R4 = L-A, A-Aromatic-Carbon
R5 = L-A, A-Aromatic-Carbon
R6 = L-A, A-Aromatic-Carbon
R7 = L-A, A-Aromatic-Carbon

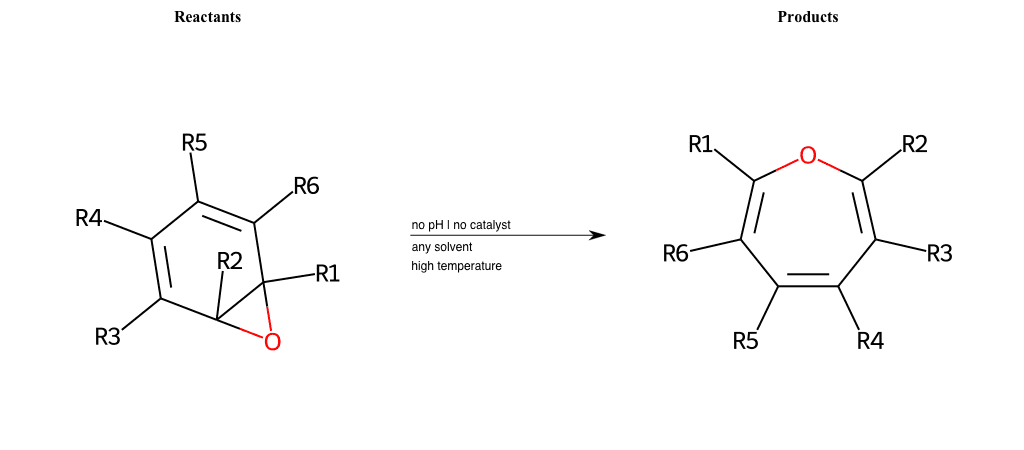
# Buchner-Ring-Expansion-O
References:
[0]
Pericyclic reaction - Wikipedia
Special Conditions : SIDE REACTION
# Ene-Reaction-C=S
References:
[0]
Ene reaction - Wikipedia
[1]
Ene Reaction
Condition to enforce:
R1 = L-A, A-Aromatic-Carbon
R2 = L-A, A-Aromatic-Carbon
R3 = L-A, A-Aromatic-Carbon
R4 = L-A, A-Aromatic-Carbon
R5 = L-A, A-Aromatic-Carbon
R6 = L-A, A-Aromatic-Carbon
R7 = L-A, A-Aromatic-Carbon

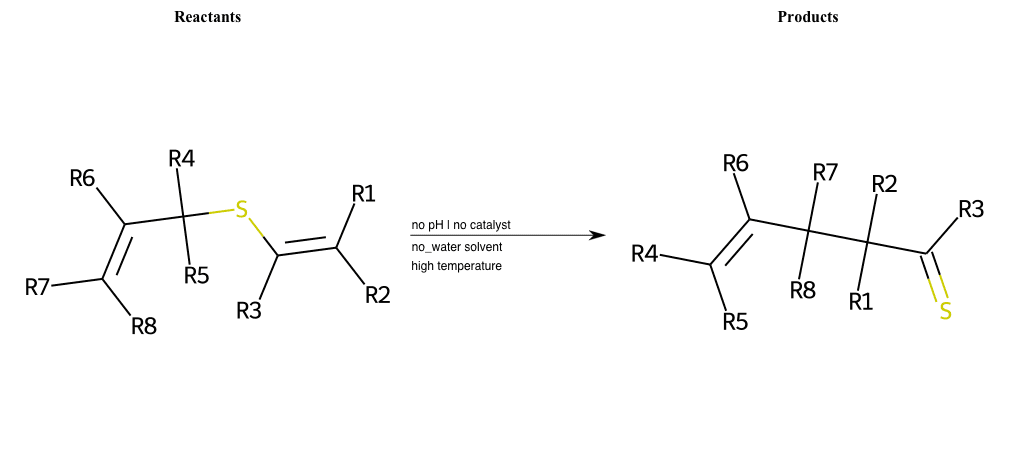
# Thio-Claisen-Rearrangement
References:
[0]
Claisen rearrangement - Wikipedia
[1]
Claisen Rearrangement
Special Conditions : SIDE REACTION
# Ene-Reaction-N=N
References:
[0]
Ene reaction - Wikipedia
[1]
Ene Reaction
Condition to enforce:
R1 = H, A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon
R4 = H, A-Aliphatic-Carbon
R5 = H, A-Aliphatic-Carbon
R6 = H, A-Aliphatic-Carbon
R7 = H, A-Aliphatic-Carbon

# 1,3-Dipolar-Cycloaddition-Type-II
References:
[0]
1,3-Dipolar cycloaddition - Wikipedia

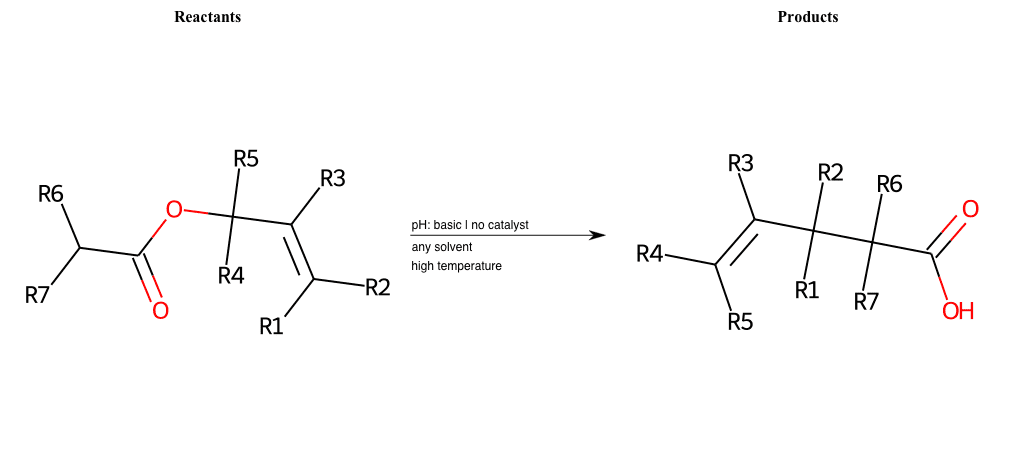
# Enolate-Claisen-Rearrangement
References:
[0]
Claisen rearrangement - Wikipedia
[1]
Claisen Rearrangement
[2]
Sigmatropic rearrangements
Special Conditions : SIDE REACTION Condition to enforce:
R1 = H, A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon
R4 = H, A-Aliphatic-Carbon
R5 = H, A-Aliphatic-Carbon
R6 = H, A-Aliphatic-Carbon
R7 = H, A-Aliphatic-Carbon
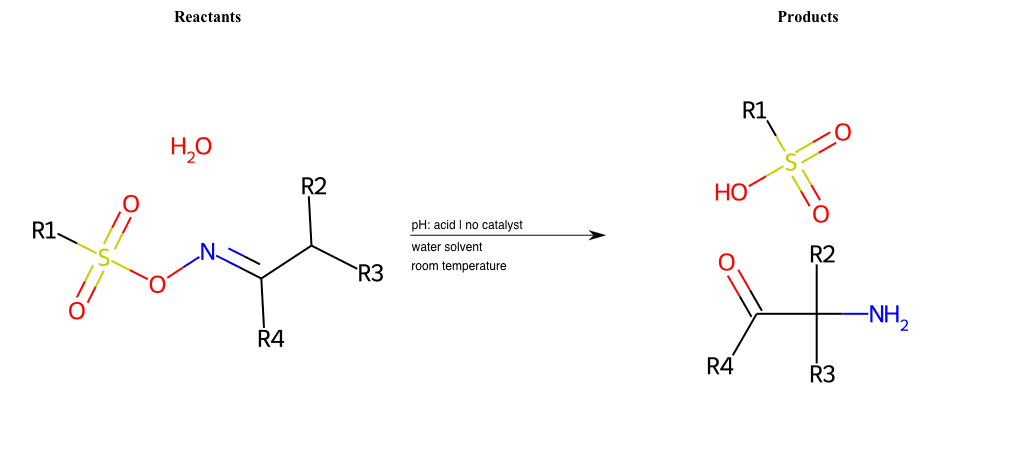
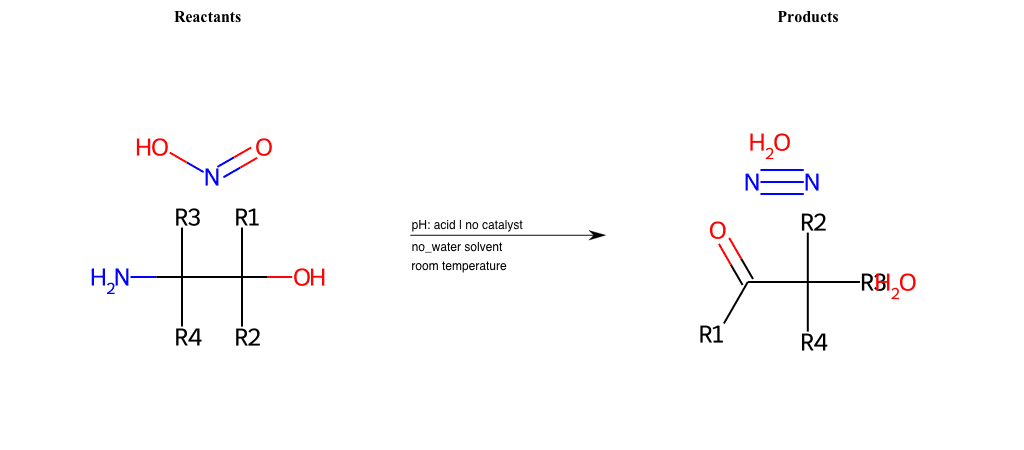
# Tiffeneau-Demjanov-Rearrangement
References:
[0]
Tiffeneau-Demjanov Rearrangement
Condition to enforce:
R1 = L-A, A-Aromatic-Carbon
R2 = L-A, A-Aromatic-Carbon
R3 = L-A, A-Aromatic-Carbon
R4 = L-A, A-Aromatic-Carbon
# Azo-Vinylcyclopropane-Rearrangement
References:
[0]
Vinylcyclopropane rearrangement - Wikipedia
Special Conditions : SIDE REACTION Condition to enforce:
R1 = L-A, A-Aromatic-Carbon
R2 = L-A, A-Aromatic-Carbon
# Diels-Alder-4+2-Oxygen
References:
[0]
diels-alder-reaction
[1]
Pi Donors And Resonance - Pi Donors Make Carbons More Nucleophilic
Condition to enforce:
R1 = H, A-Aliphatic-Carbon
R2 = L-A
R3 = L-A
R4 = H, A-Aliphatic-Carbon
R5 = L-A
R6 = L-A
R7 = L-A
R8 = L-A
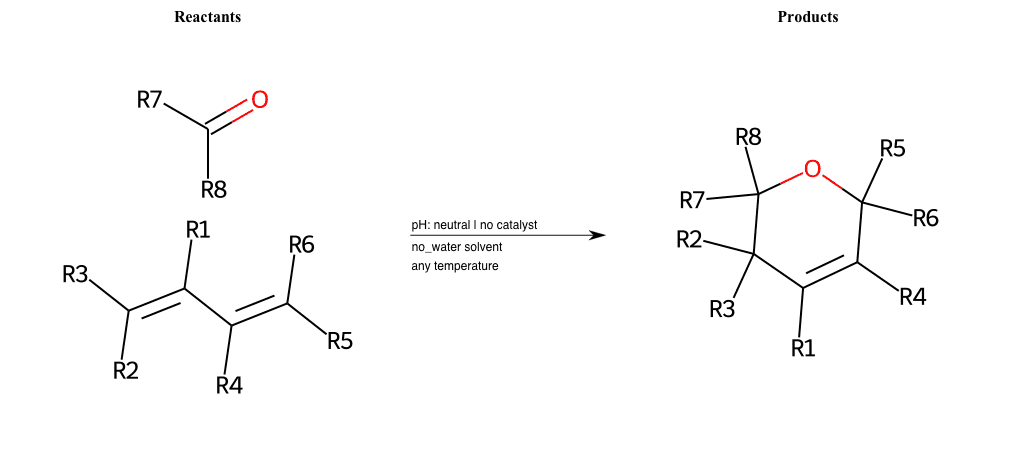
# Azo-Cope
References:
[0]
Aza-Cope/Mannich reaction - Organic Reactions Wiki
Special Conditions : SIDE REACTION Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = L-A
R7 = L-A
R8 = L-A
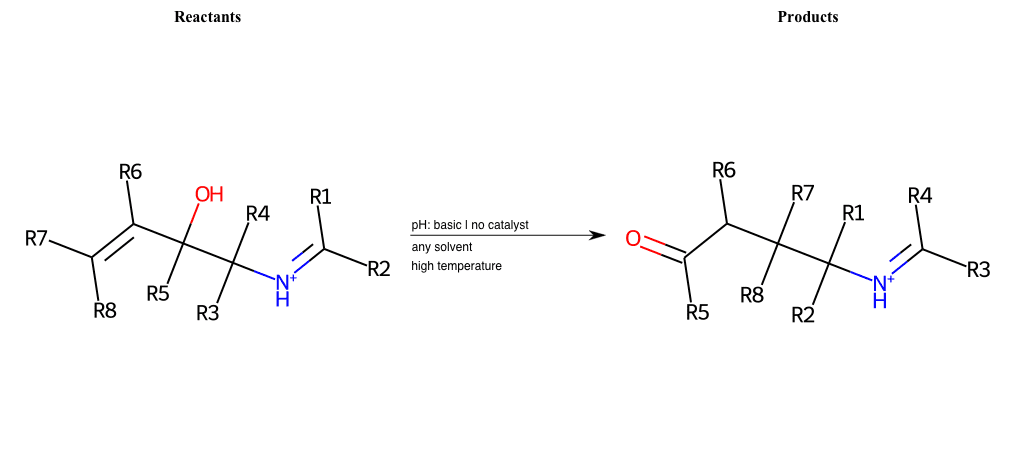
# Conia-Ene-Reaction
References:
[0]
Conia-Ene Reaction
Condition to enforce:
R1 = L-A, A-Aromatic-Carbon
R2 = L-A, A-Aromatic-Carbon
R3 = L-A, A-Aromatic-Carbon
R4 = L-A, A-Aromatic-Carbon
R5 = L-A, A-Aromatic-Carbon
R6 = L-A, A-Aromatic-Carbon
R7 = L-A, A-Aromatic-Carbon
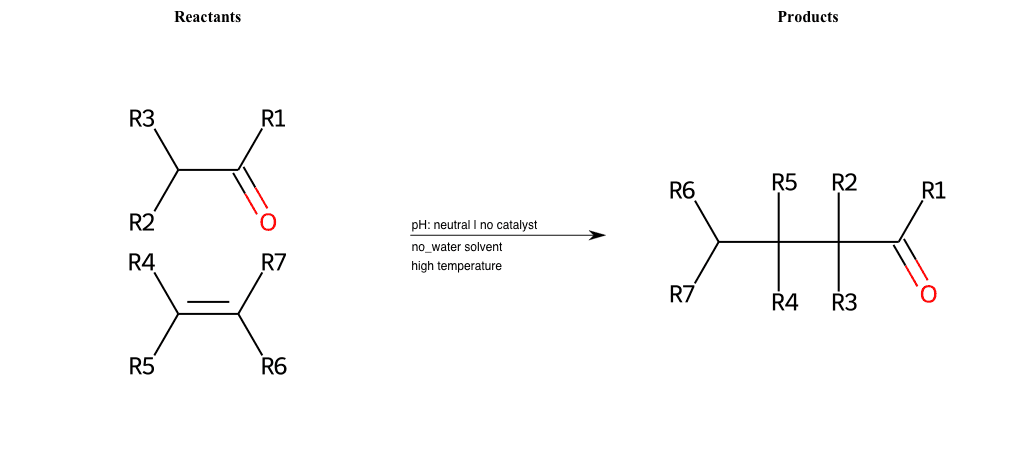
# Favorskii-Rearrangement-Br
References:
[0]
Favorskii rearrangement - Wikipedia
[1]
favorskii-rearrangement-1.html
Condition to enforce:
R1 = L-A, A-Aromatic-Carbon
R2 = L-A, A-Aromatic-Carbon
R3 = L-A, A-Aromatic-Carbon
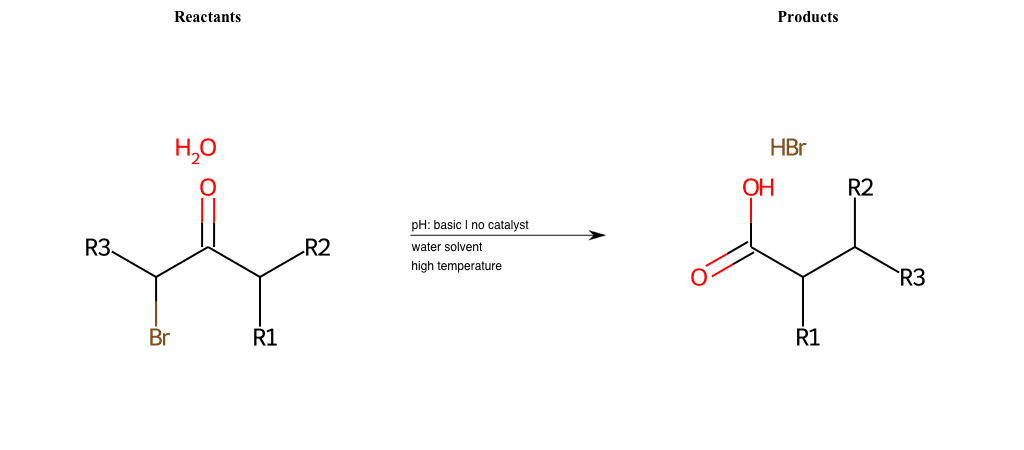
# Overman-Rearrangement-Pt2
References:
[0]
Overman Rearrangement
[1]
Overman Rearrangement
Special Conditions : SIDE REACTION Condition to enforce:
R1 = H, A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon