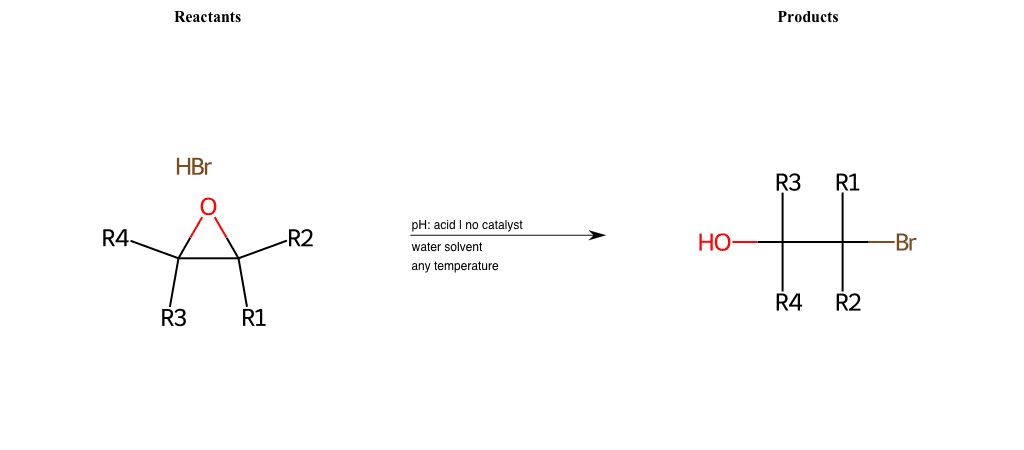
Nucleophilic-Aliphatic-Substitutions
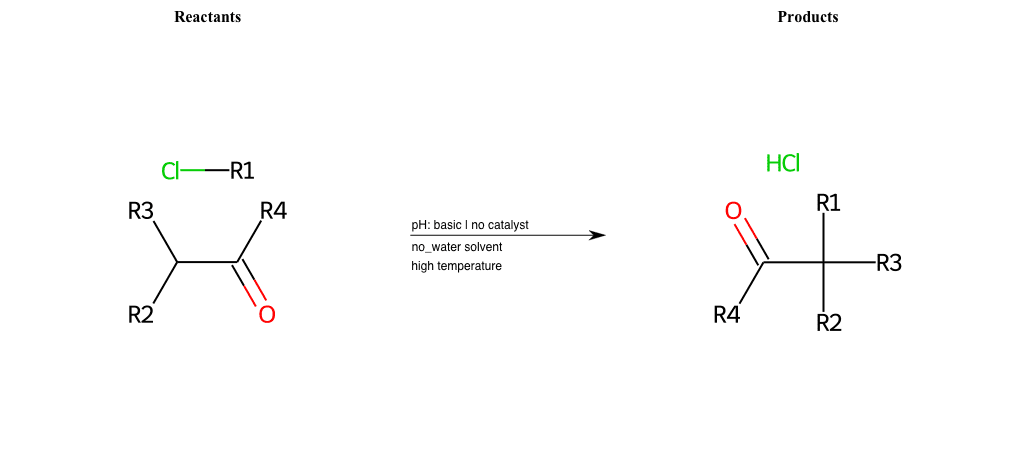
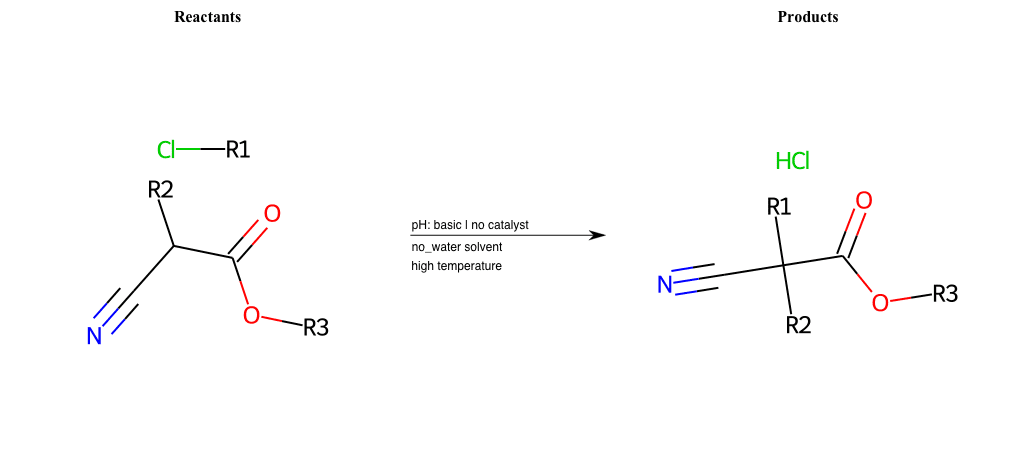
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Alkane-and-EWG2-Carbonyl-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon
R4 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
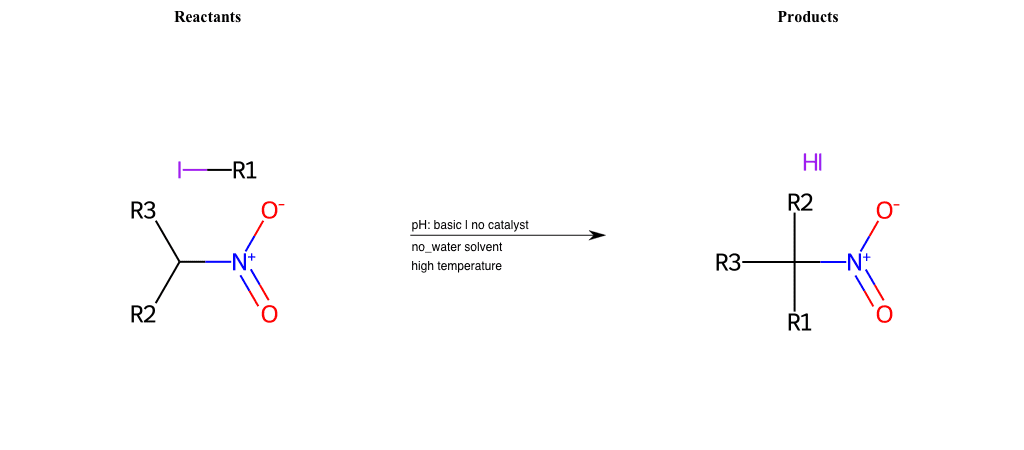
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Alkane-and-EWG2-Nitrite-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon
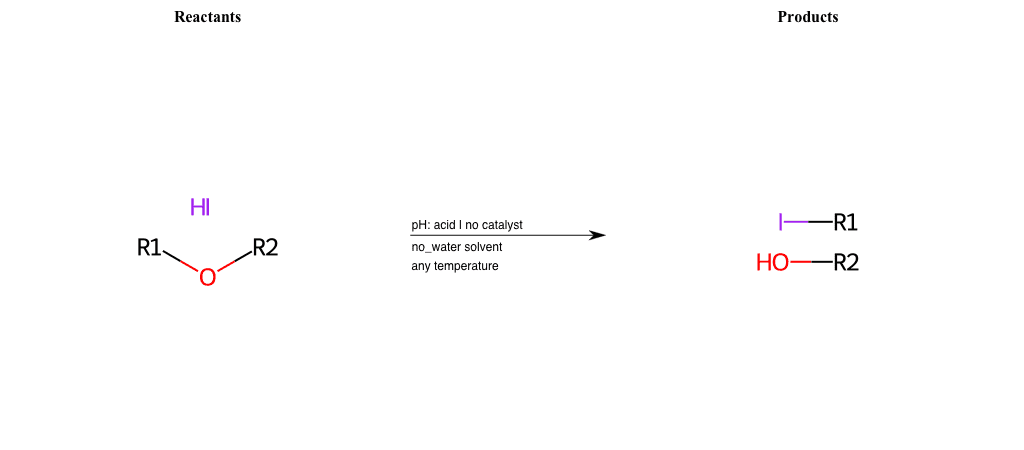
# Nucleophilic-Aliphatic-Substitution-Acidic-Lg-Alkoxide-and-Nu-Iodine
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Acidic cleavage of ethers (SN2) – Master Organic Chemistry
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = A-Aliphatic-Carbon
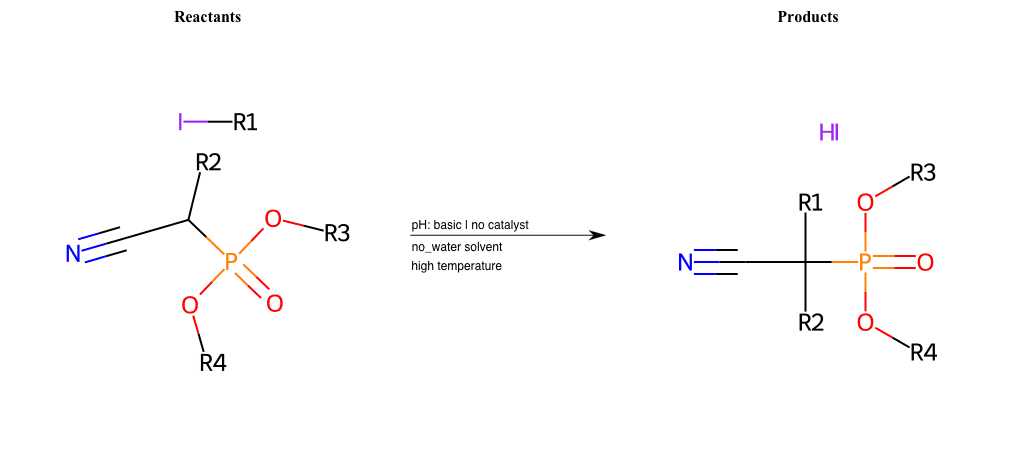
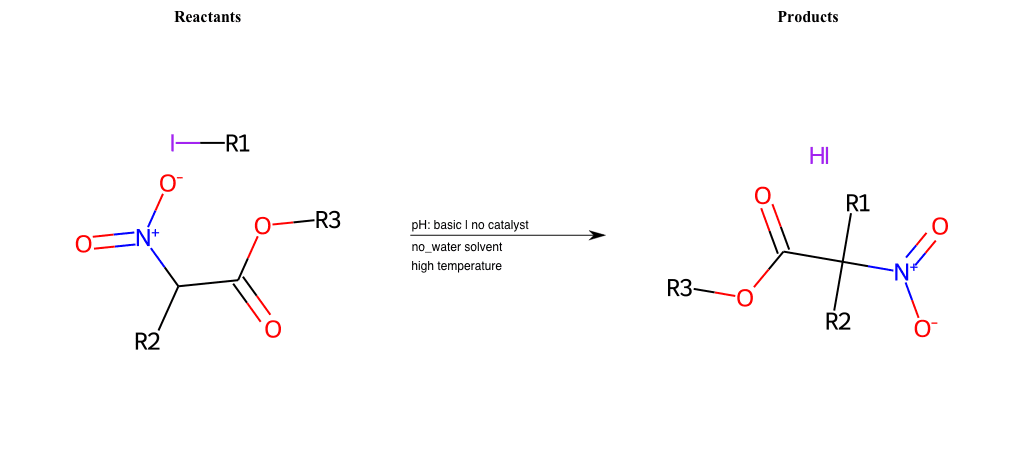
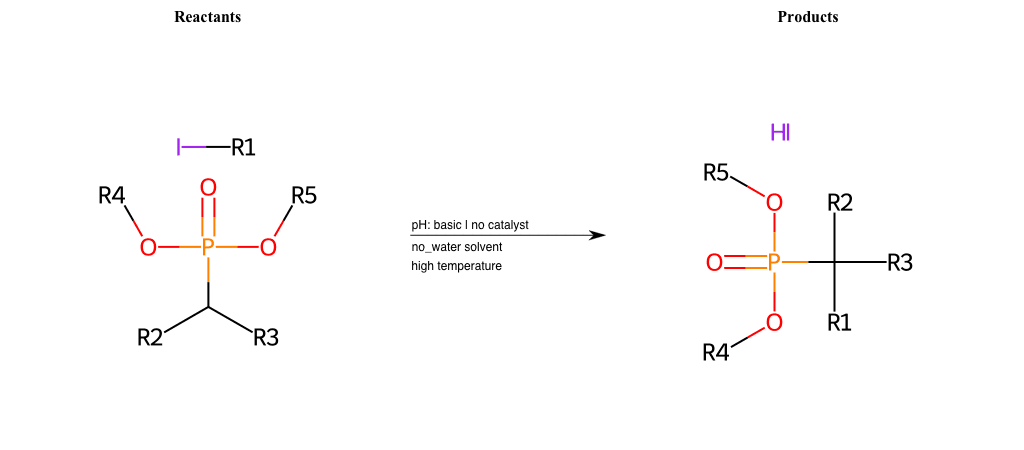
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Nitrile-and-EWG2-Phosphonate-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon
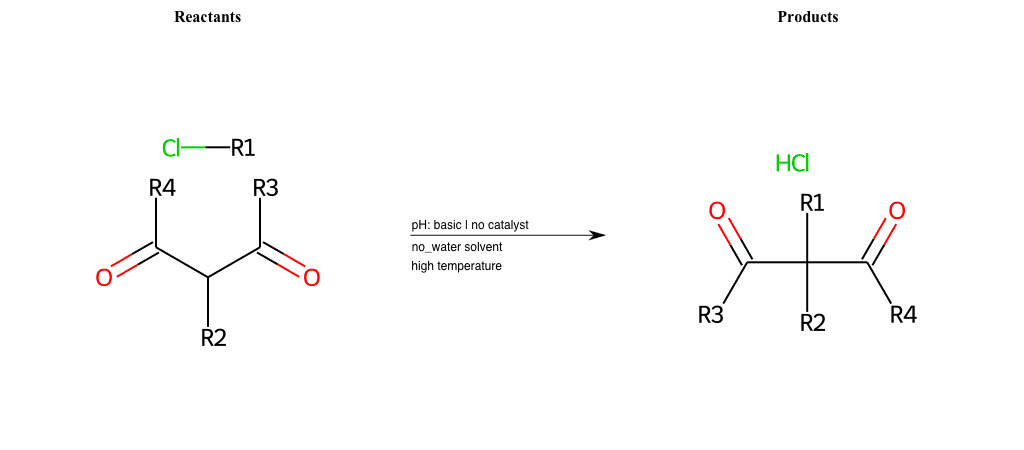
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carbonyl-and-EWG2-Carboxyl-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
# Epoxide-Ring-Opening-Nu-Thiolate-acid
References:
[0]
Epoxidation - Chemistry LibreTexts
[1]
Epoxides Ring-Opening Reactions - Chemistry Steps
Condition to enforce:
R1 = L-A, A-Aromatic-Carbon
R2 = L-A, A-Aromatic-Carbon
R3 = L-A, A-Aromatic-Carbon
R4 = L-A, A-Aromatic-Carbon
R5 = A-Aliphatic-Carbon, A-Aromatic-Carbon
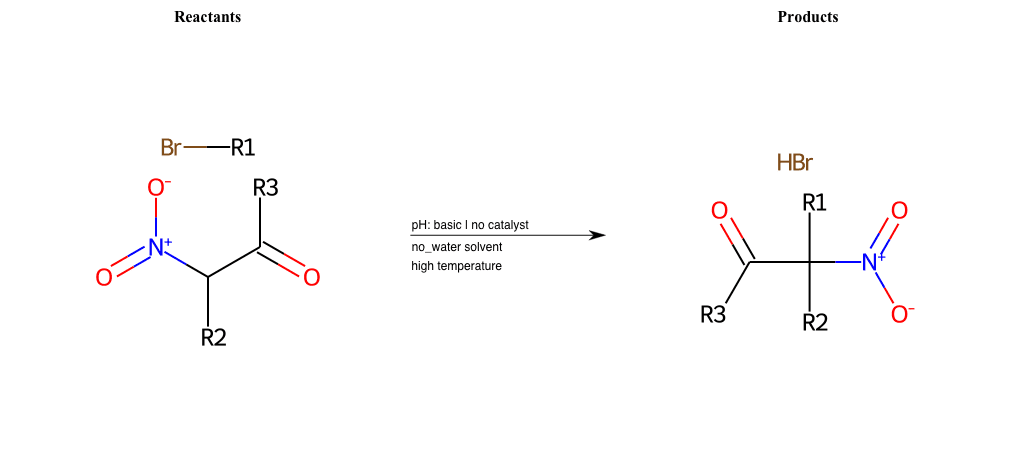
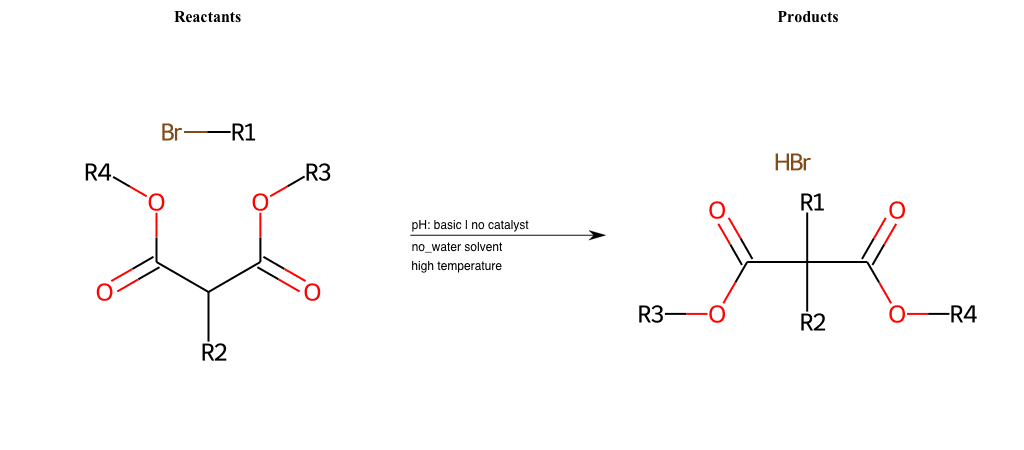
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carbonyl-and-EWG2-Carbonyl-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R4 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
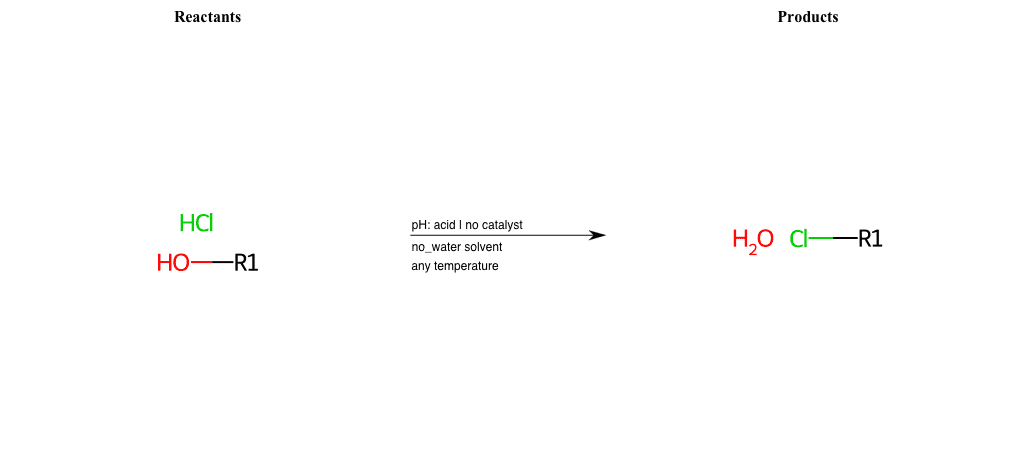
# Nucleophilic-Aliphatic-Substitution-Acidic-Lg-Hydroxyl-and-Nu-Chlorine
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Making Alkyl Halides From Alcohols – Master Organic Chemistry
[4]
Ch15 : Alcohols with hydrogen halides to alkyl halides
Condition to enforce:
R1 = A-Aliphatic-Carbon
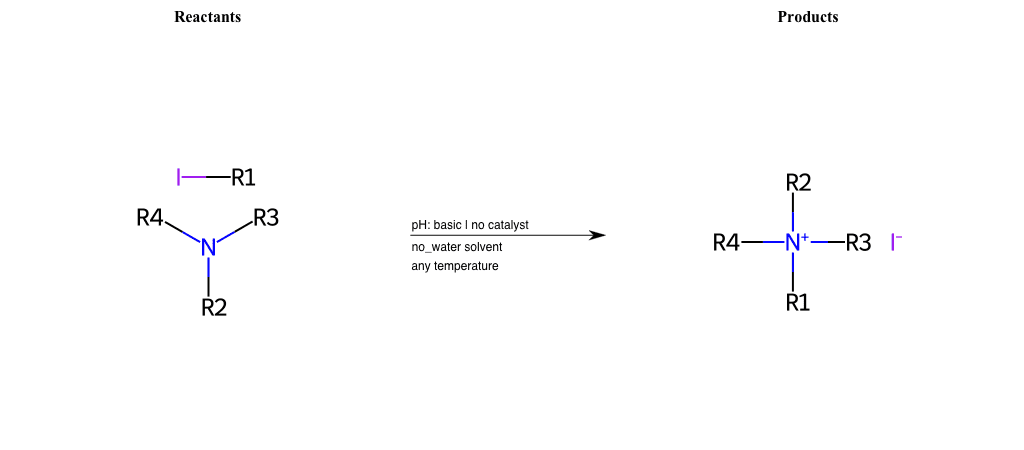
# Nucleophilic-Aliphatic-Substitution-Tertiary-Amine-Lg-Iodine
References:
[0]
Alkylation of Amines (Sucks!) – Master Organic Chemistry
[1]
9.4. Reaction of RX with NH3 and amines | Organic Chemistry 1: An open textbook
[2]
Amines as Nucleophiles - Chemistry LibreTexts
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R3 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon
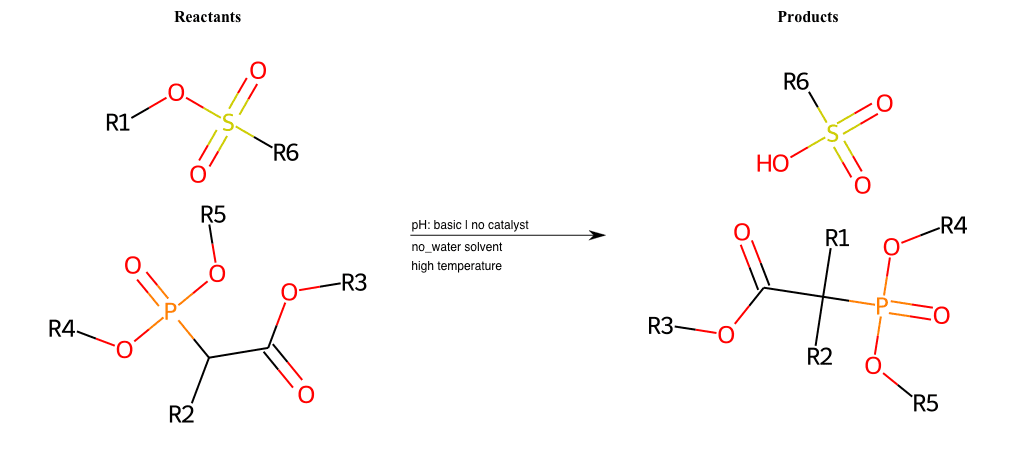
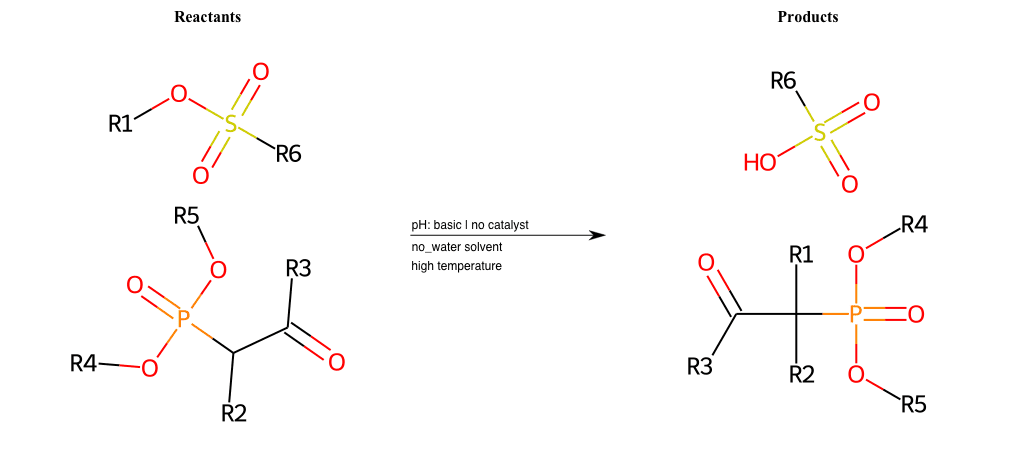
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carboxyl-and-EWG2-Phosphonate-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R5 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
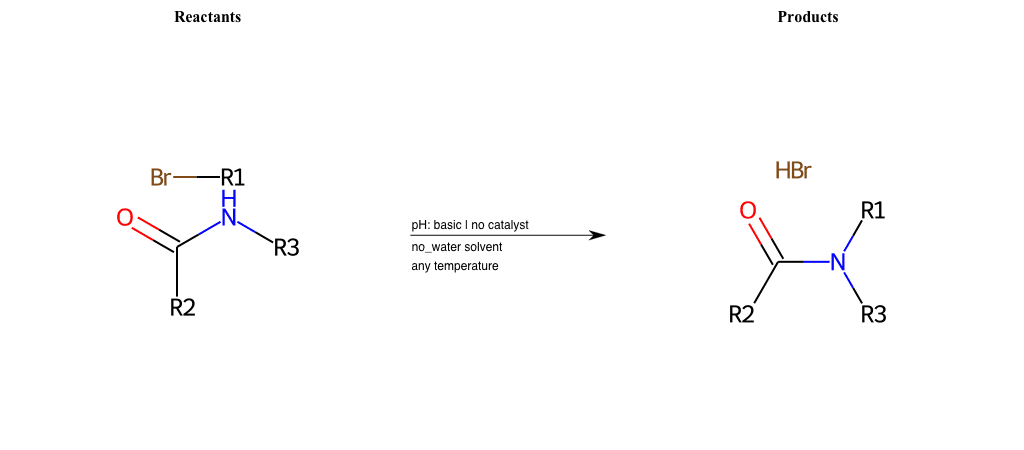
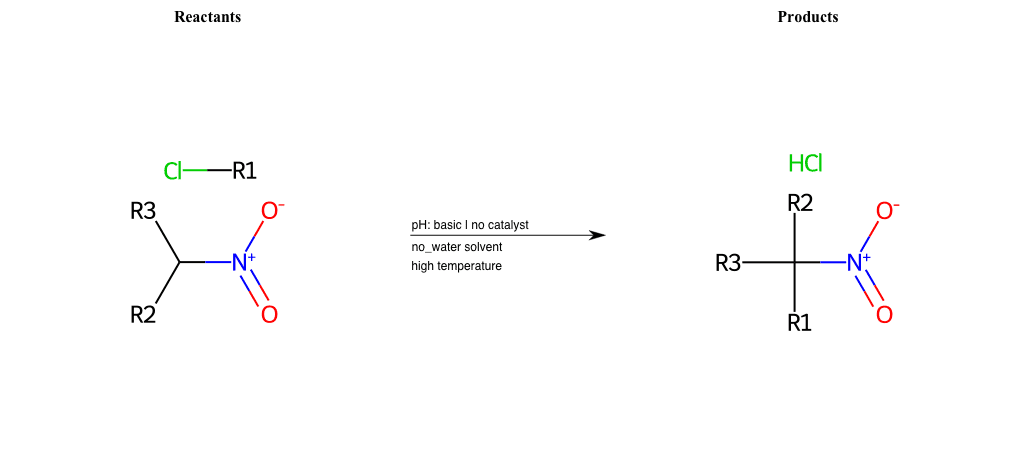
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Bromine-and-Nu-N-Amide
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Nitrile-and-EWG2-Nitrite-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
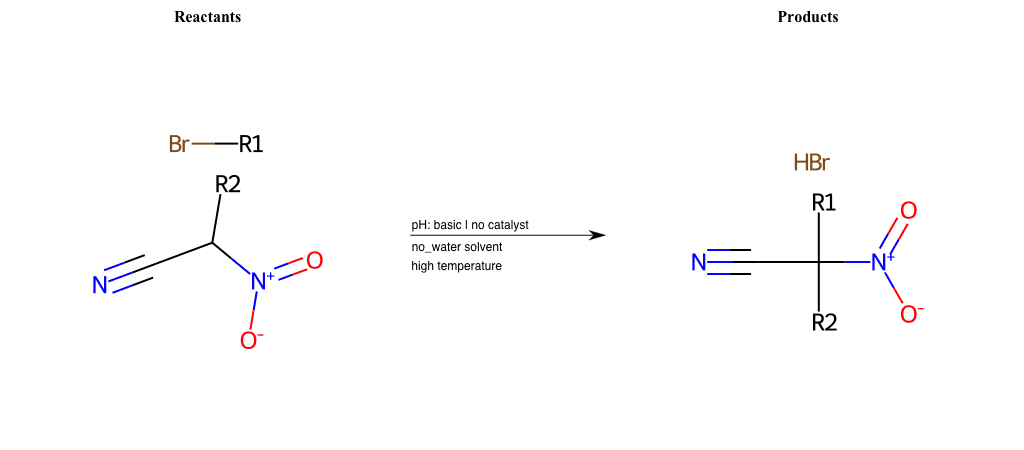
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Nitrite-and-EWG2-Phosphonate-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon
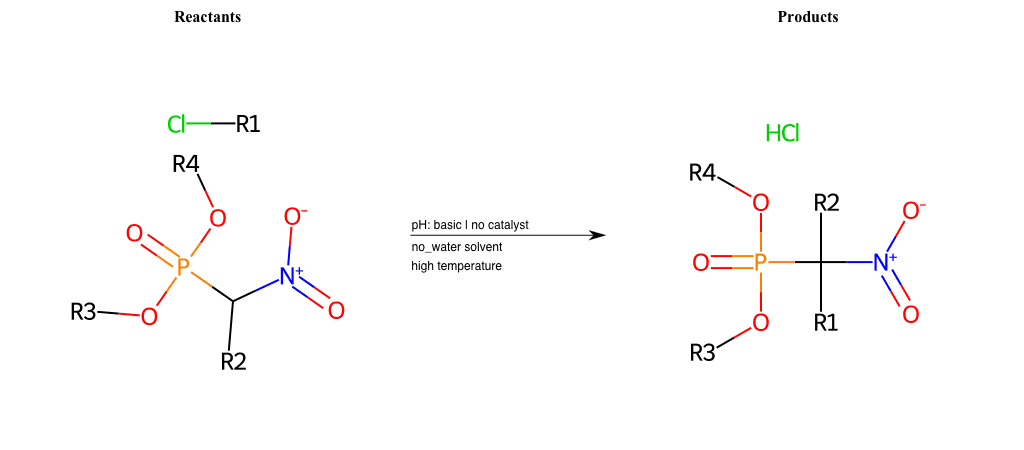
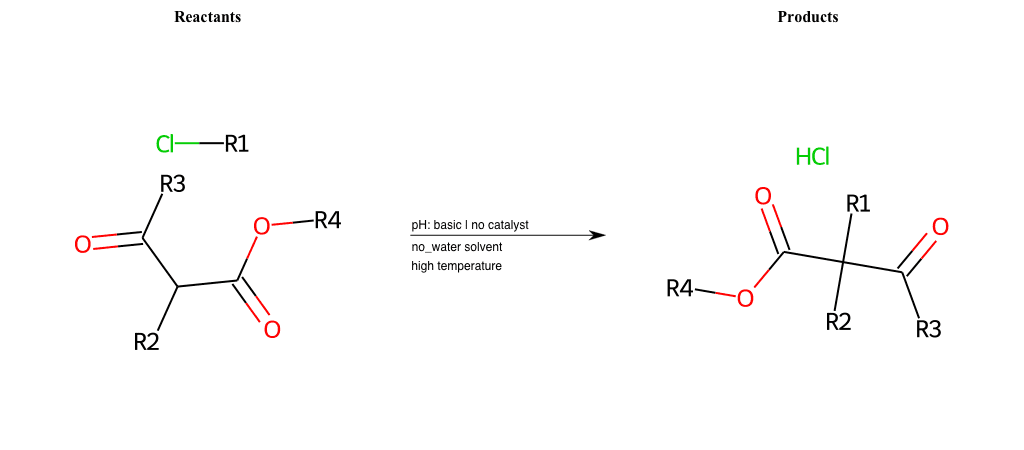
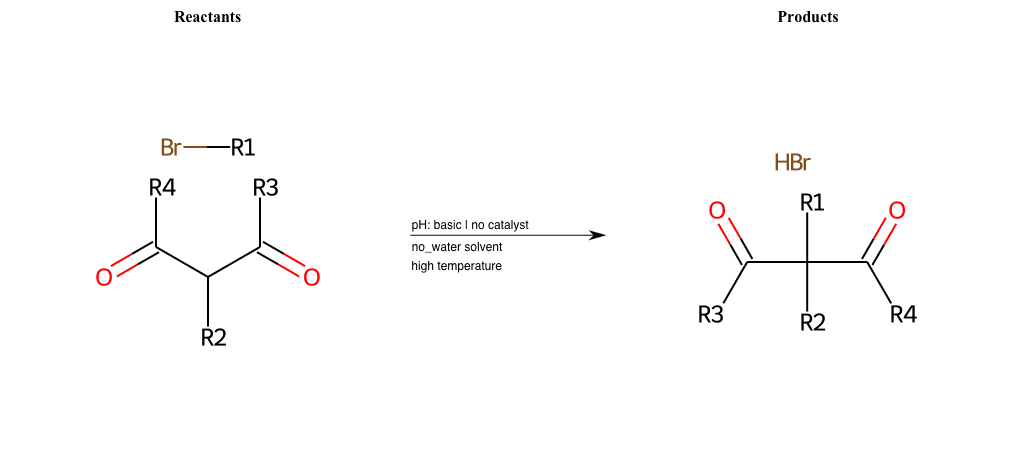
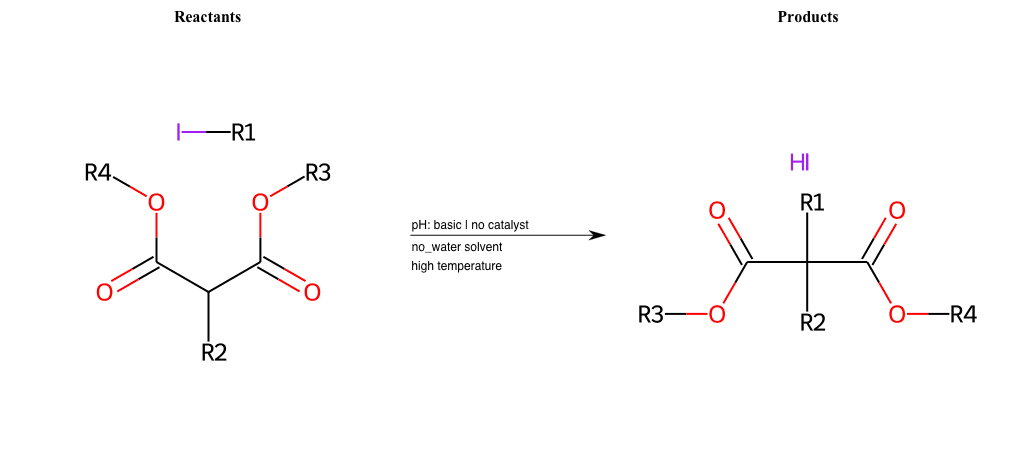
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carboxyl-and-EWG2-Carboxyl-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
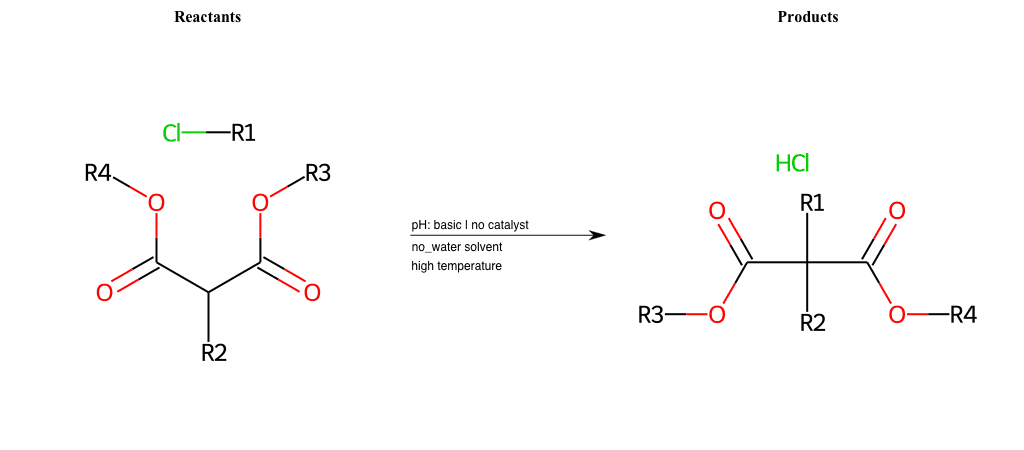
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Bromine-and-Nu-Amino
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Alkylation of Amines (Sucks!) – Master Organic Chemistry
[4]
9.4. Reaction of RX with NH3 and amines | Organic Chemistry 1: An open textbook
[5]
Amines as Nucleophiles - Chemistry LibreTexts
[6]
Gabriel Phthalimide Synthesis Mechanism - Explanation and Examples
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
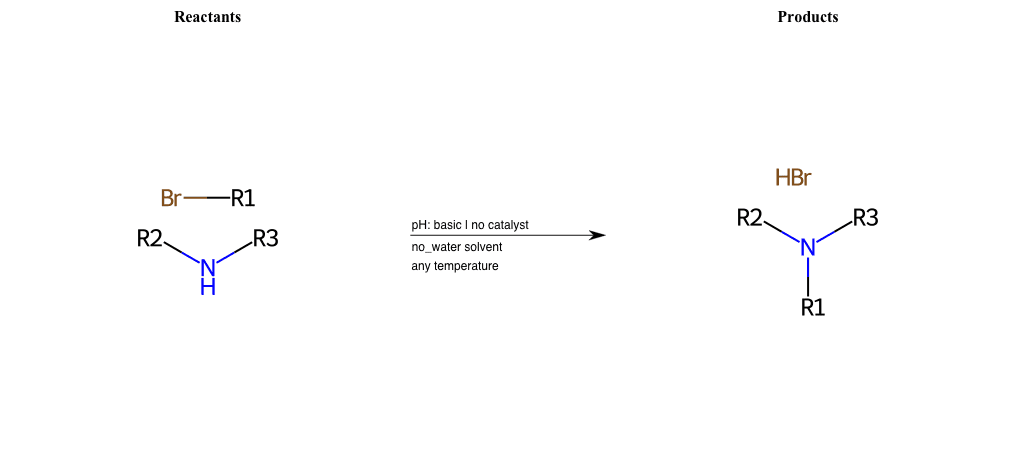
# Nucleophilic-Aliphatic-Substitution-Acidic-Lg-Sulfonate-and-Nu-Iodine
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
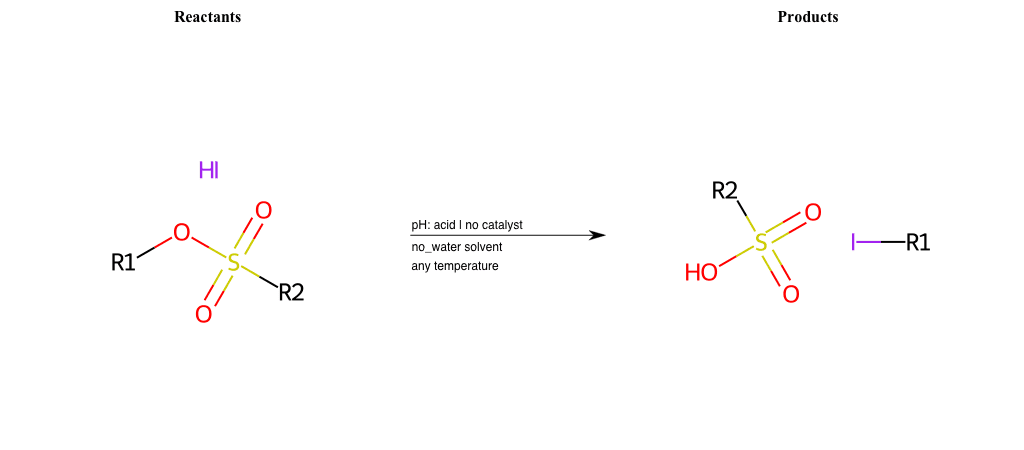
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Iodine-and-Nu-Thiolate
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = A-Aliphatic-Carbon, A-Aromatic-Carbon
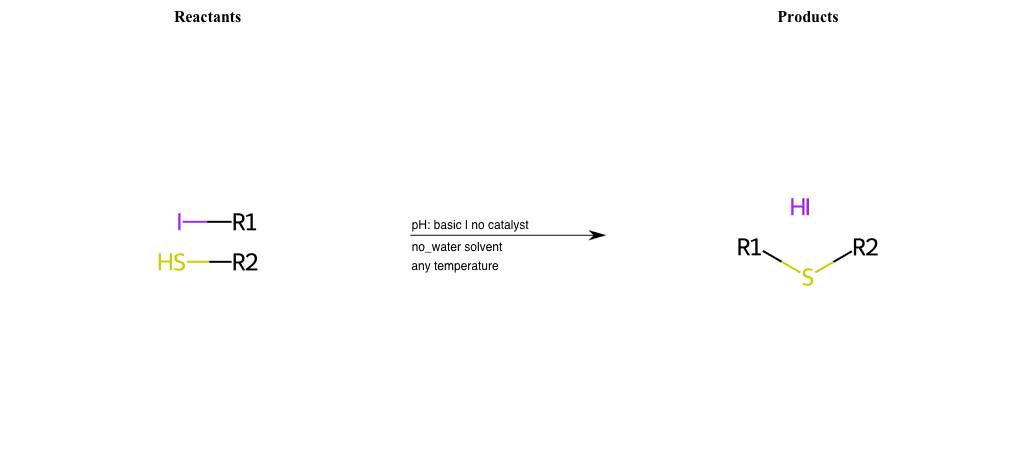
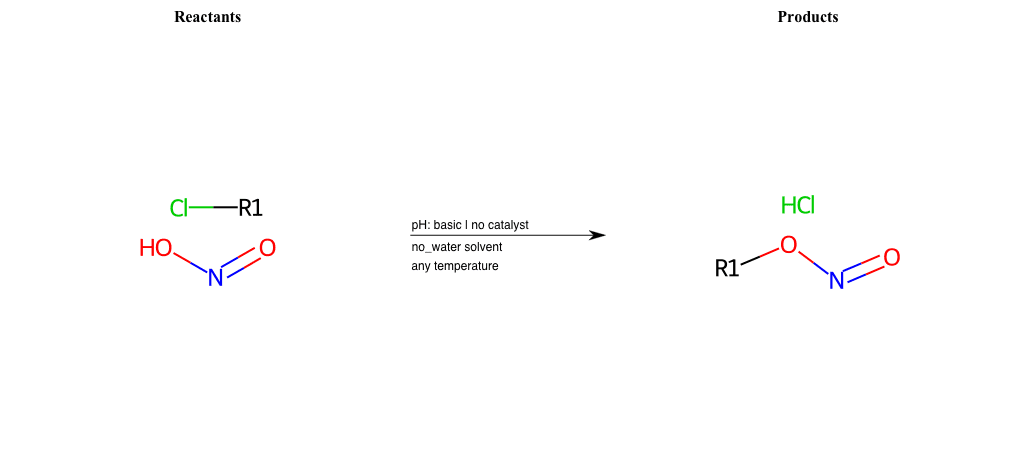
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Chlorine-and-Nu-ONO
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Haloalkanes react with KNO2 to form alkyl nitrites while AgNO2 forms nitroalkanes as the
chief product.
Condition to enforce:
R1 = A-Aliphatic-Carbon
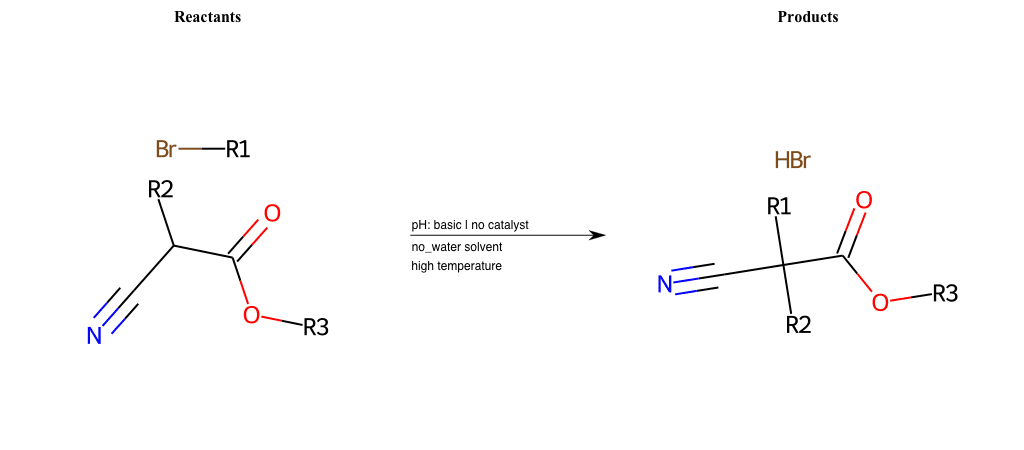
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carboxyl-and-EWG2-Nitrile-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Iodine-and-Nu-Sulfonamide
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
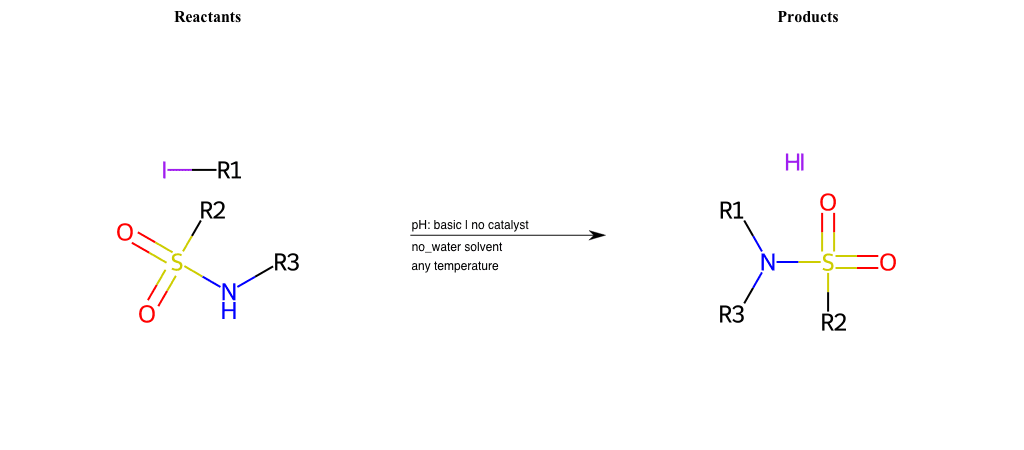
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Iodine-and-Nu-N-Amide
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
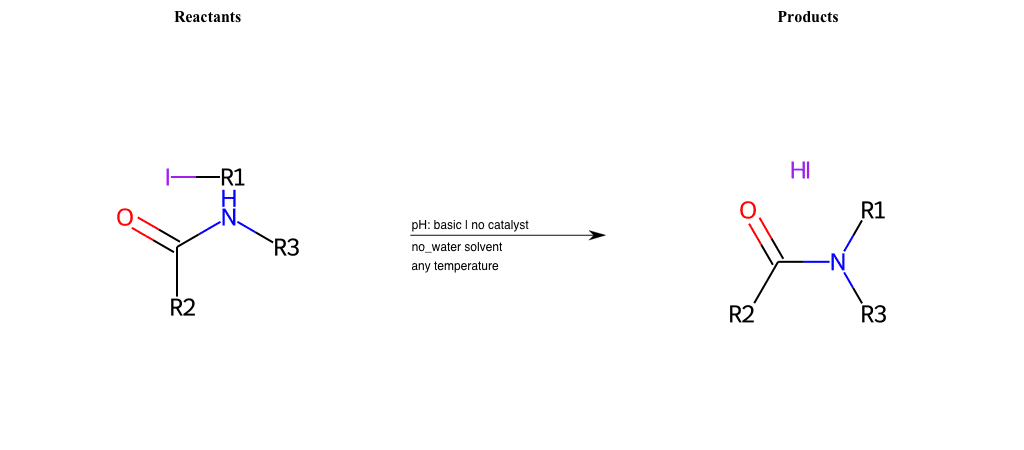
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Sulfate-and-Nu-Nitrile
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Kolbe Nitrile Synthesis
[4]
Pelouze Synthesis
Condition to enforce:
R1 = A-Aliphatic-Carbon
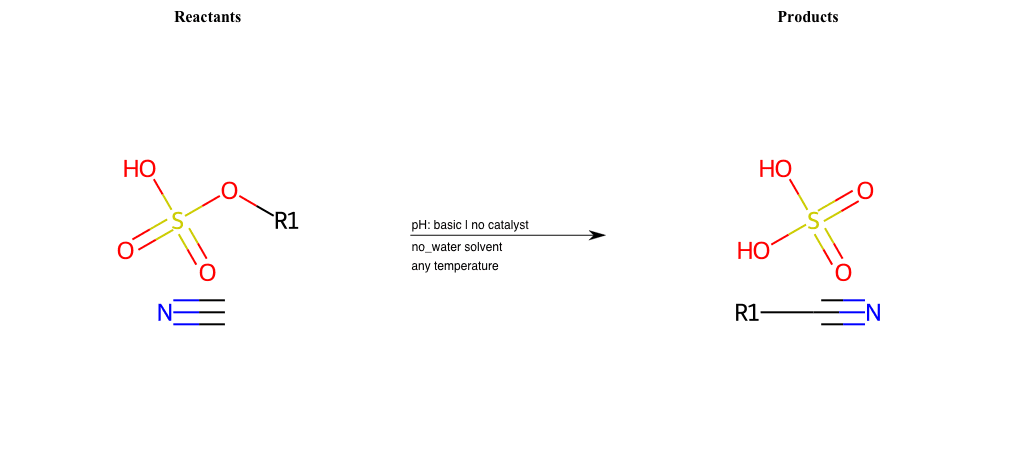
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Sulfonate-and-Nu-N-Amide
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
R4 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
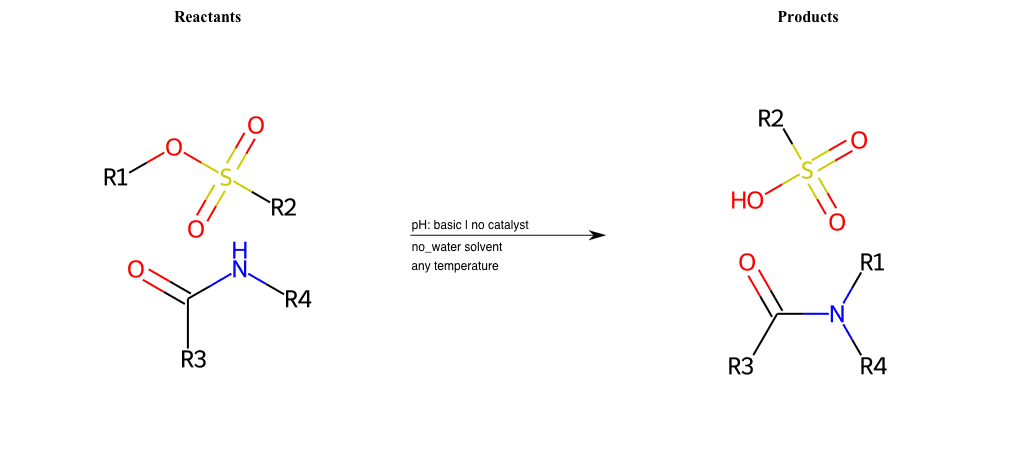
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Sulfonate-and-Nu-N-Carbamate
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
R4 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
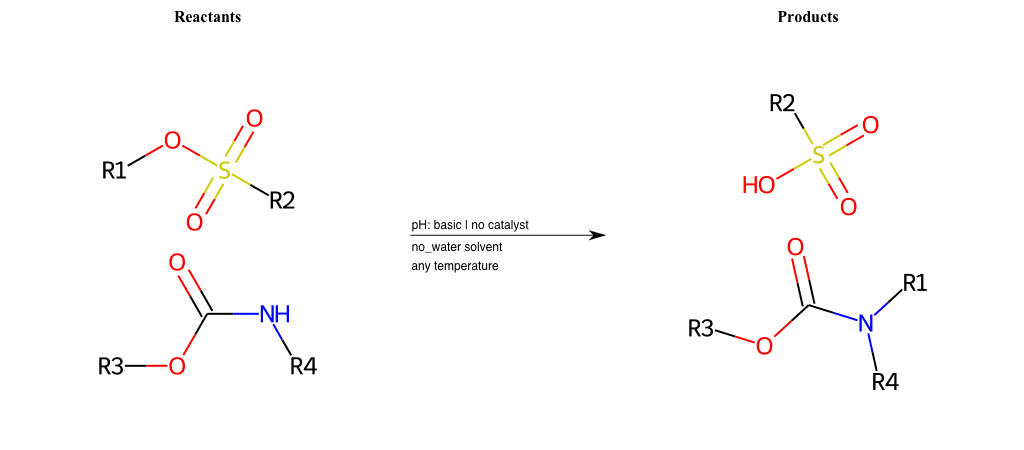
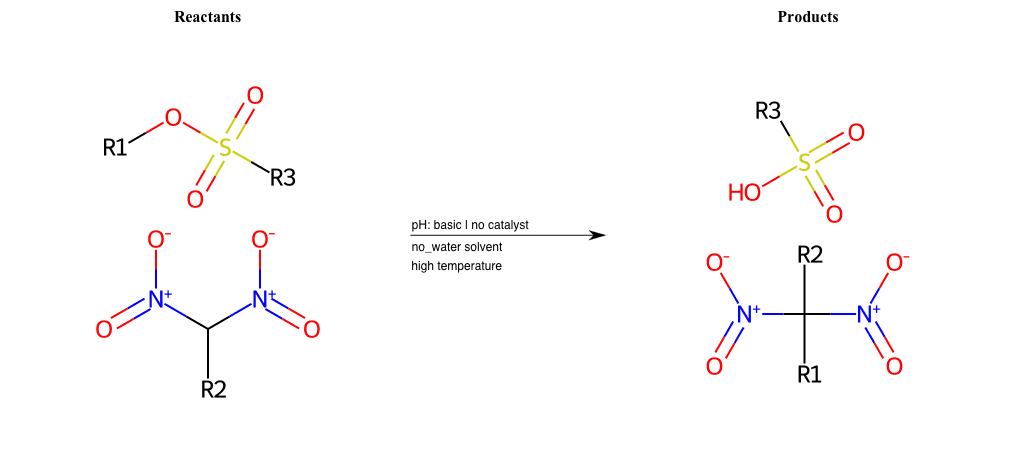
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Nitrite-and-EWG2-Phosphonate-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R5 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
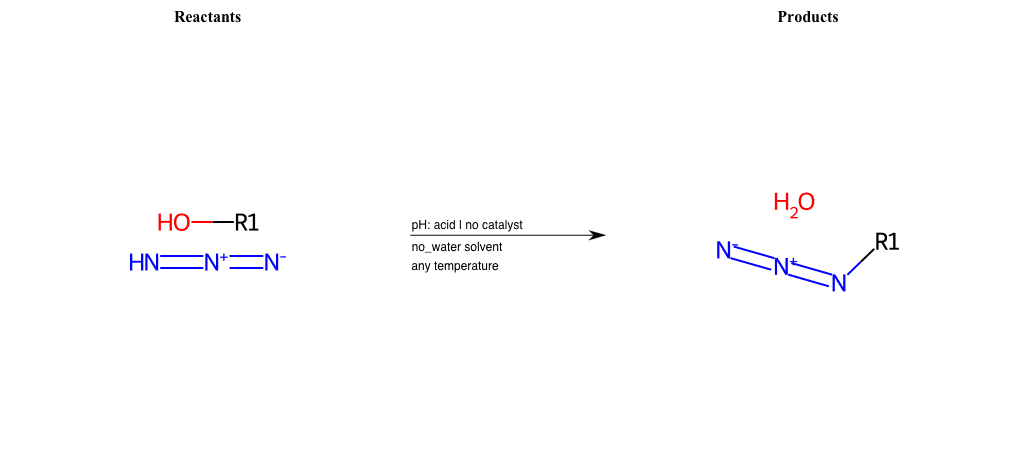
# Nucleophilic-Aliphatic-Substitution-Acidic-Lg-Hydroxyl-and-Nu-Azide
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Reactions of Azides - Substitution, Reduction, Rearrangements, and More
Condition to enforce:
R1 = A-Aliphatic-Carbon
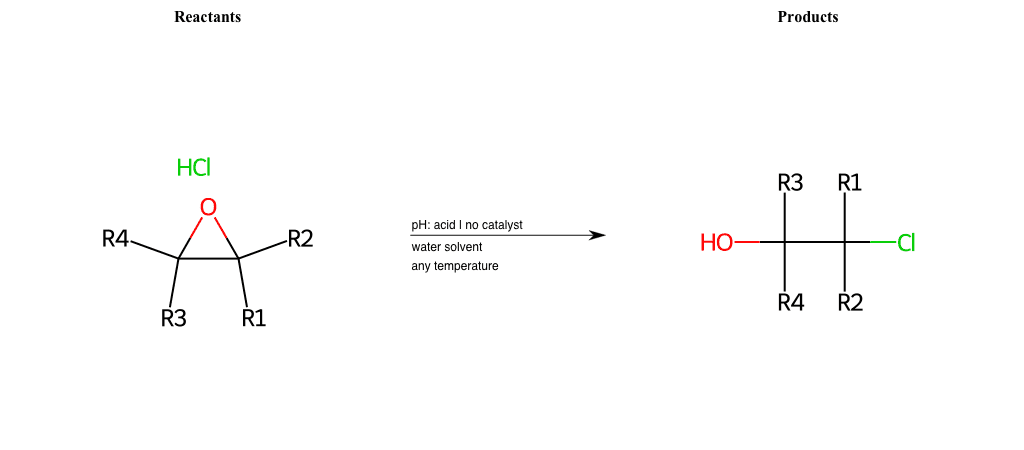
# Epoxide-Ring-Opening-Nu-Chlorine-acid
References:
[0]
Epoxidation - Chemistry LibreTexts
[1]
Epoxides Ring-Opening Reactions - Chemistry Steps
Condition to enforce:
R1 = L-A, A-Aromatic-Carbon
R2 = L-A, A-Aromatic-Carbon
R3 = L-A, A-Aromatic-Carbon
R4 = L-A, A-Aromatic-Carbon
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Chlorine-and-Nu-O-Carbamate
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
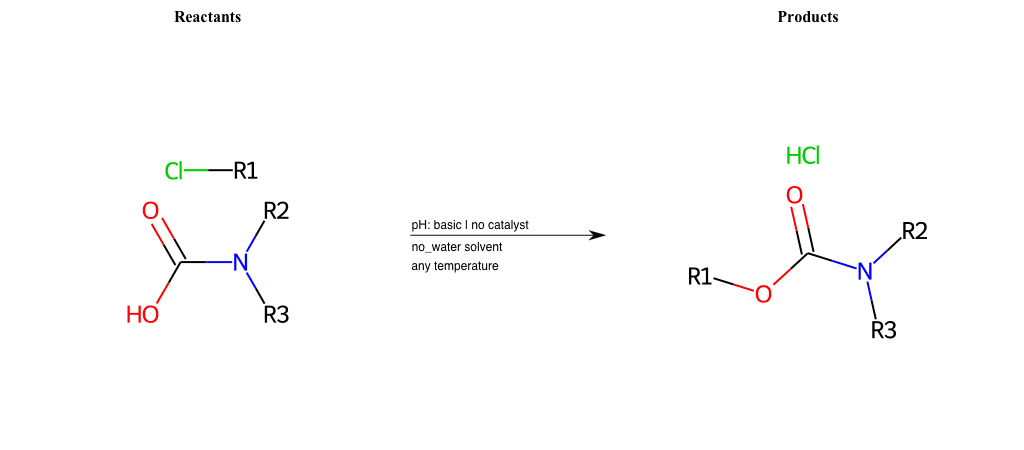
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Nitrite-and-EWG2-Nitrite-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
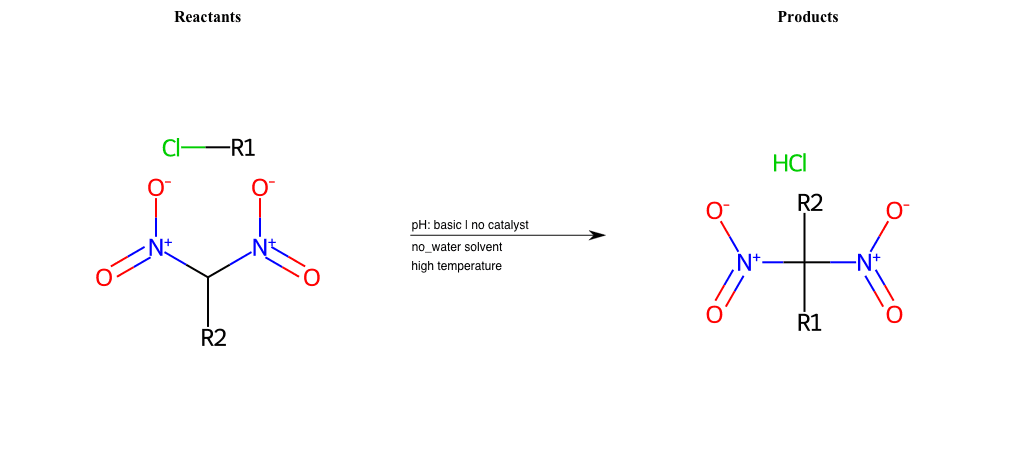
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carbonyl-and-EWG2-Carbonyl-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R4 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
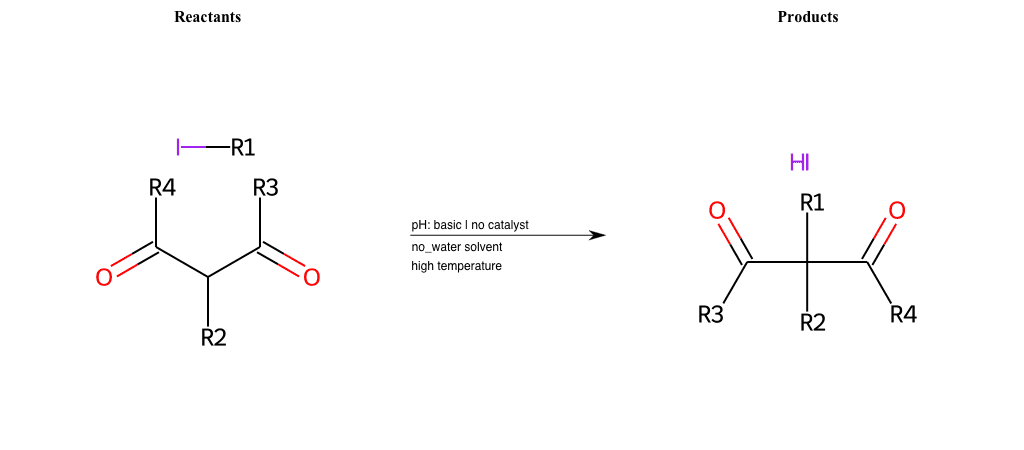
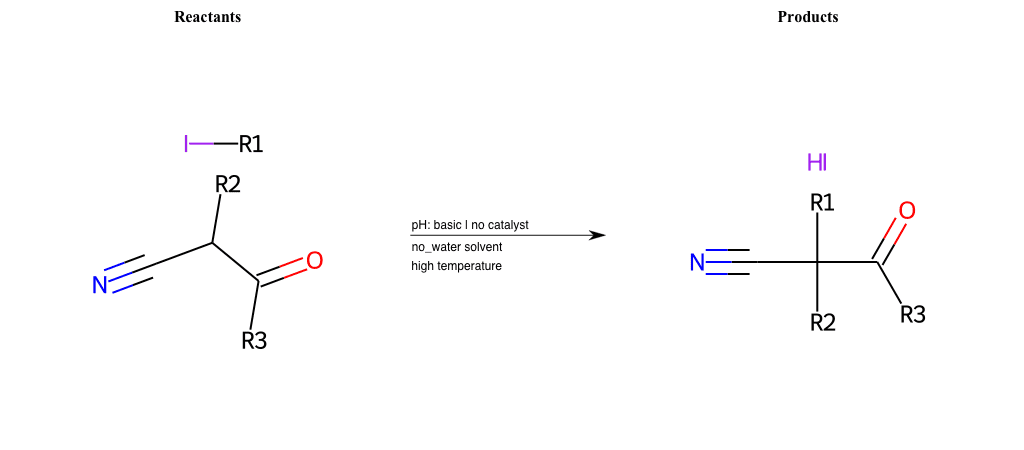
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Nitrile-and-EWG2-Nitrile-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
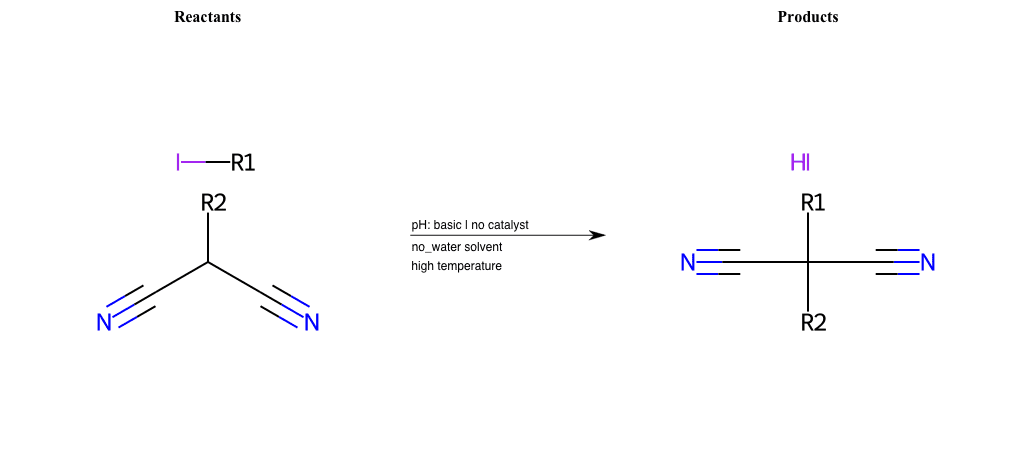
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Sulfonate-and-Nu-Azide
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Reactions of Azides - Substitution, Reduction, Rearrangements, and More
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
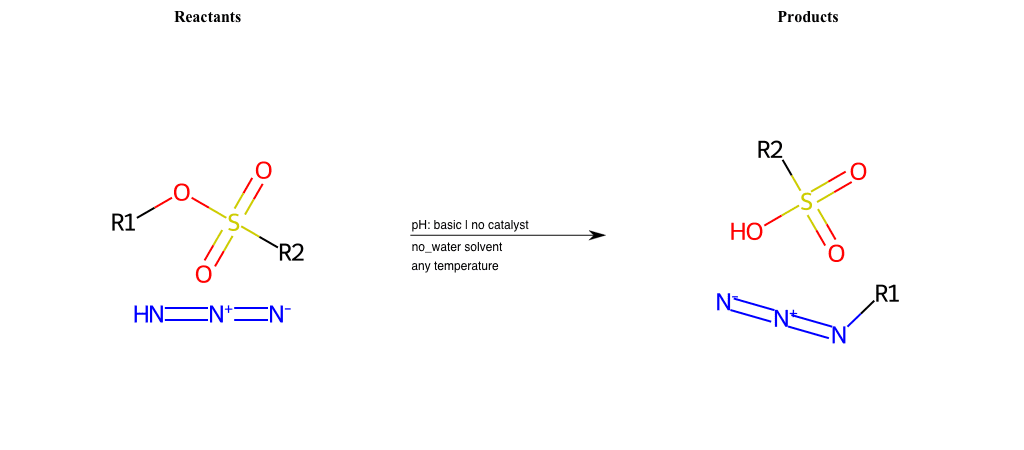
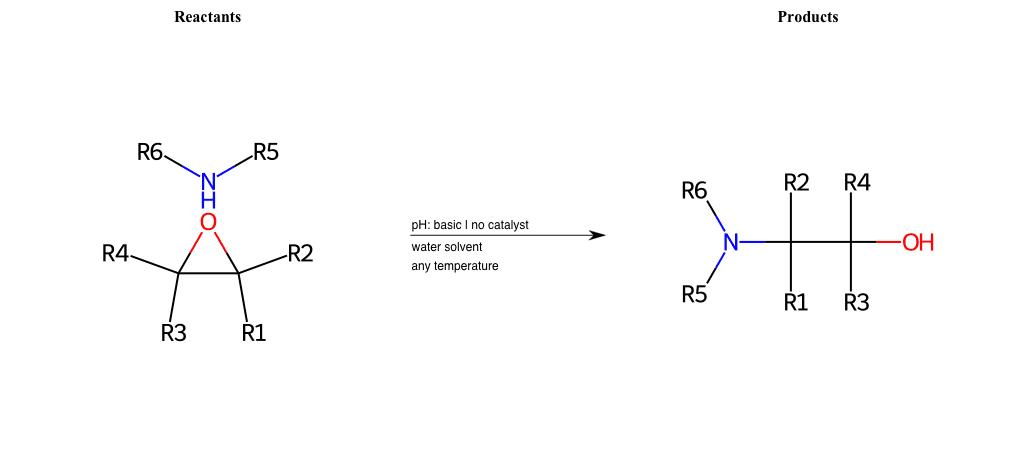
# Epoxide-Ring-Opening-Nu-Amino-basic
References:
[0]
Epoxidation - Chemistry LibreTexts
[1]
Epoxides Ring-Opening Reactions - Chemistry Steps
Condition to enforce:
R1 = L-A, A-Aromatic-Carbon
R2 = L-A, A-Aromatic-Carbon
R3 = L-A, A-Aromatic-Carbon
R4 = L-A, A-Aromatic-Carbon
R5 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
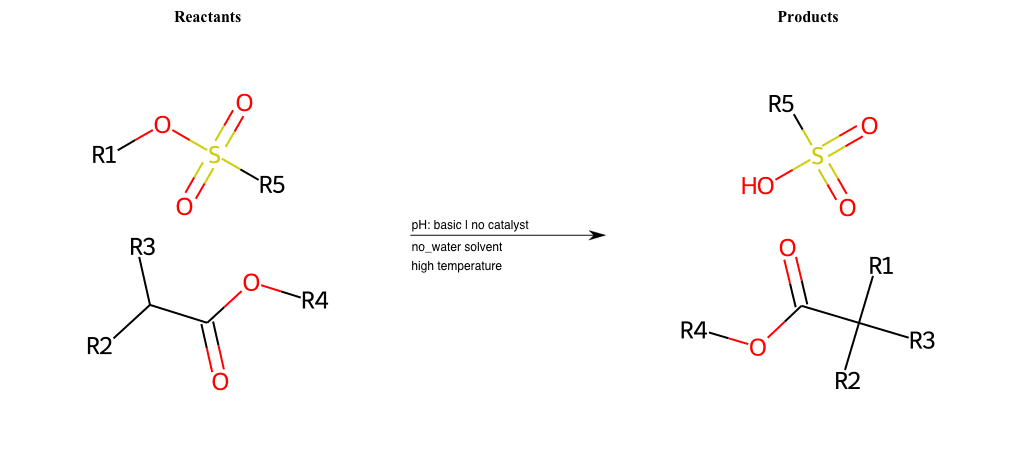
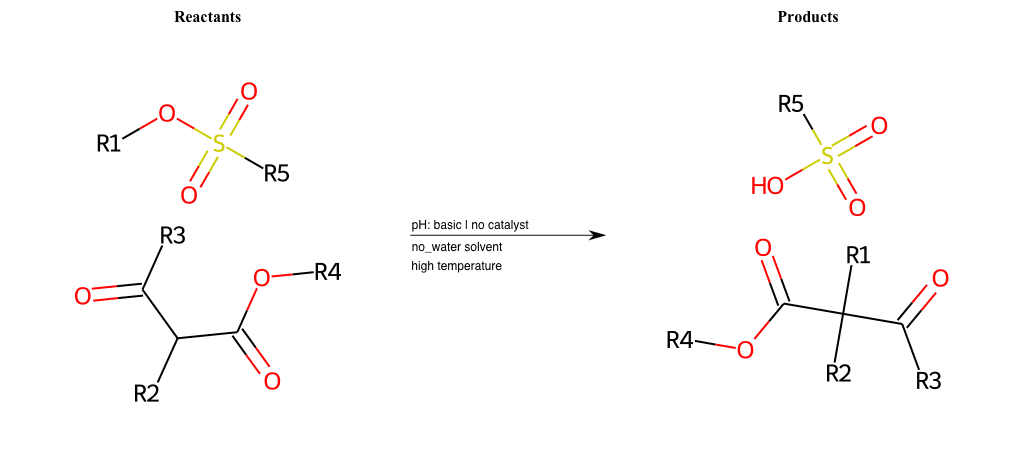
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Alkane-and-EWG2-Carboxyl-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
R5 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
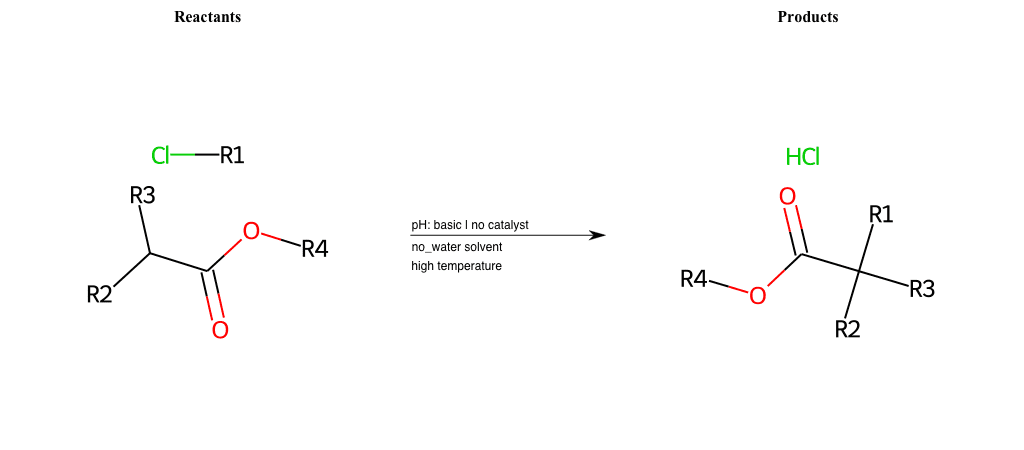
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Alkane-and-EWG2-Carboxyl-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
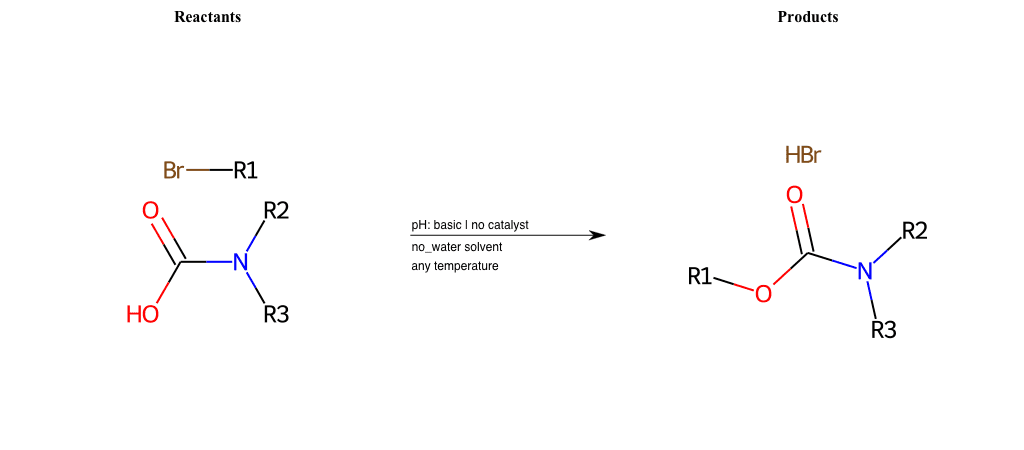
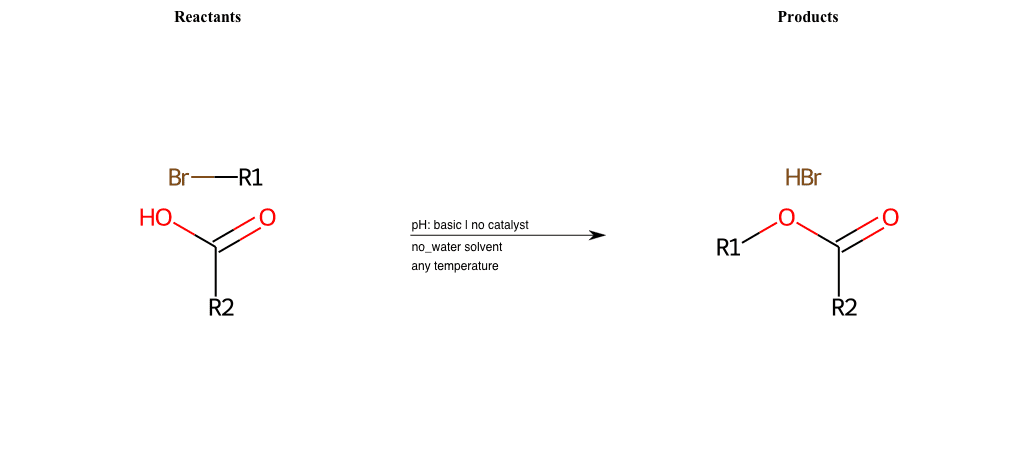
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Chlorine-and-Nu-Carboxyl-H
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
carboxylic-acid-as-nucleophile
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
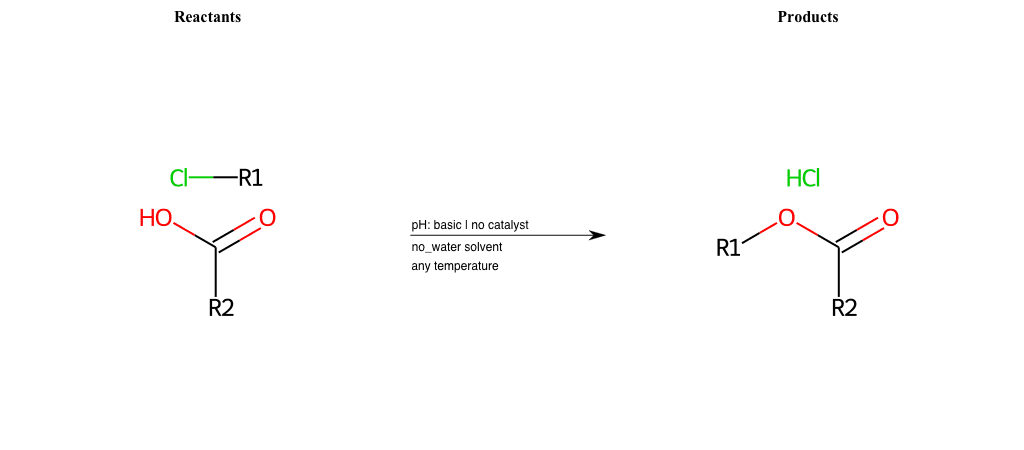
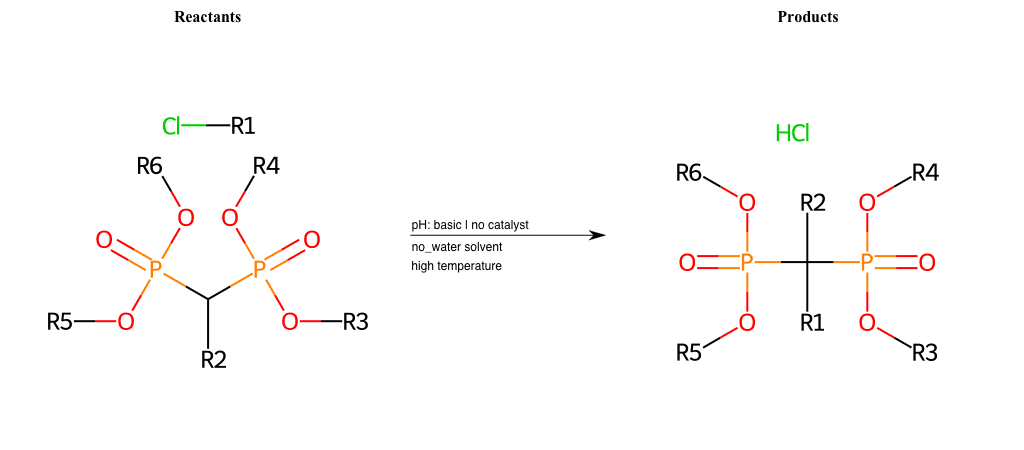
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Phosphonate-and-EWG2-Phosphonate-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R5 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R6 = A-Aliphatic-Carbon, A-Aromatic-Carbon
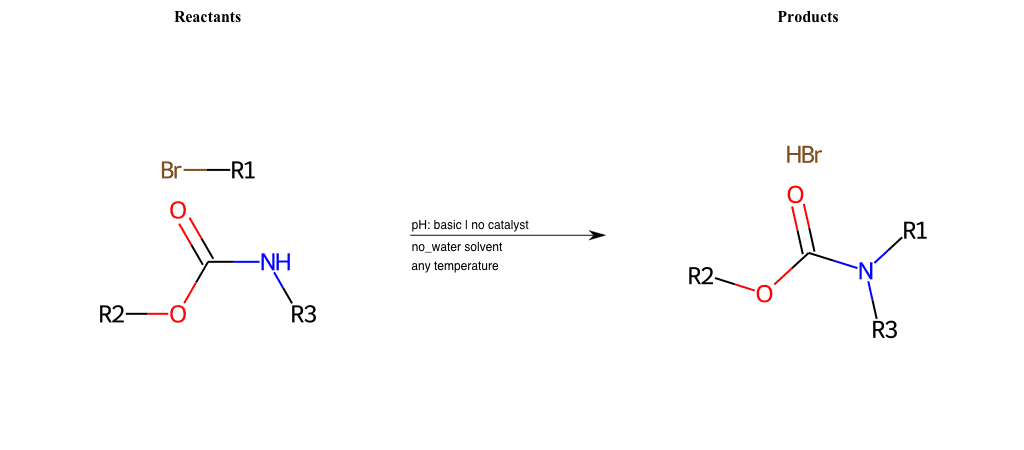
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Bromine-and-Nu-N-Carbamate
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
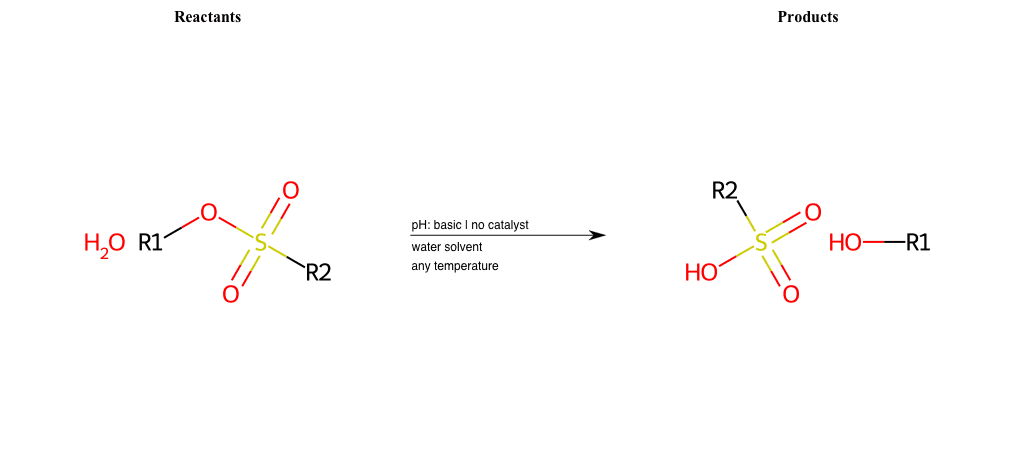
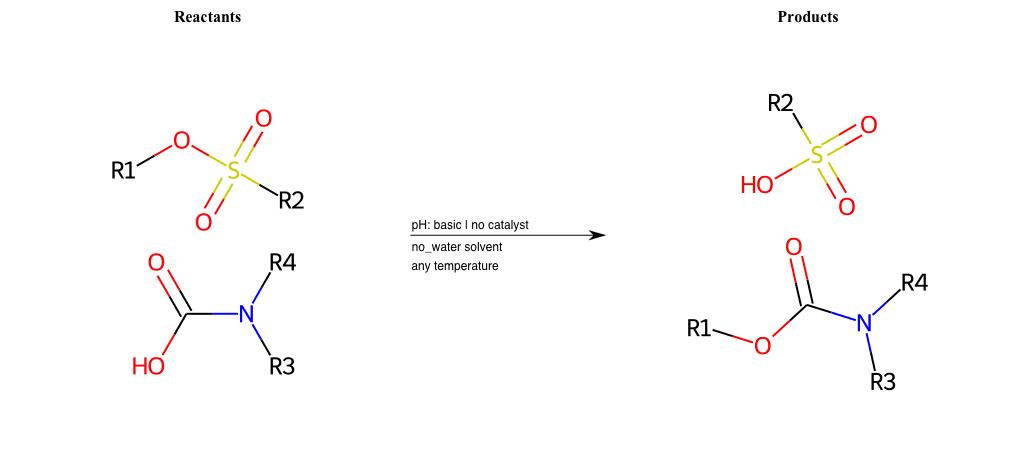
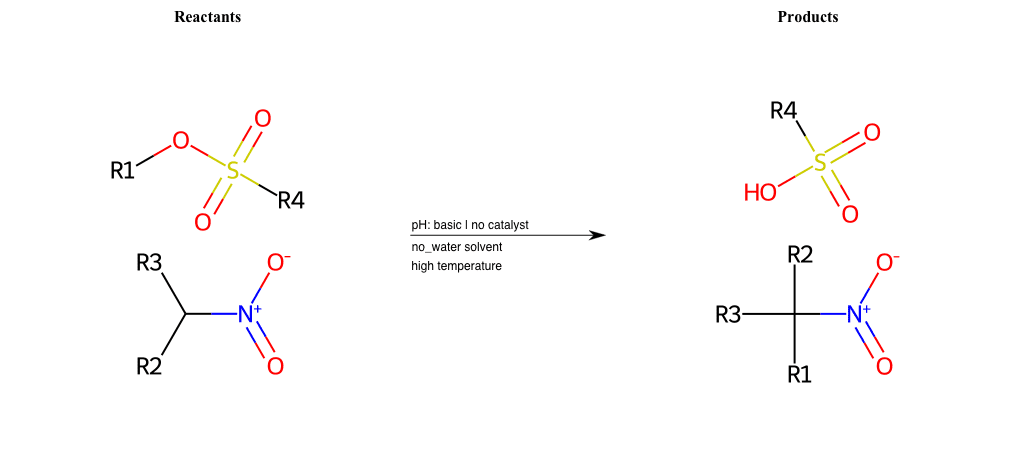
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Sulfonate-and-Nu-Hydroxyl
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carbonyl-and-EWG2-Carboxyl-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
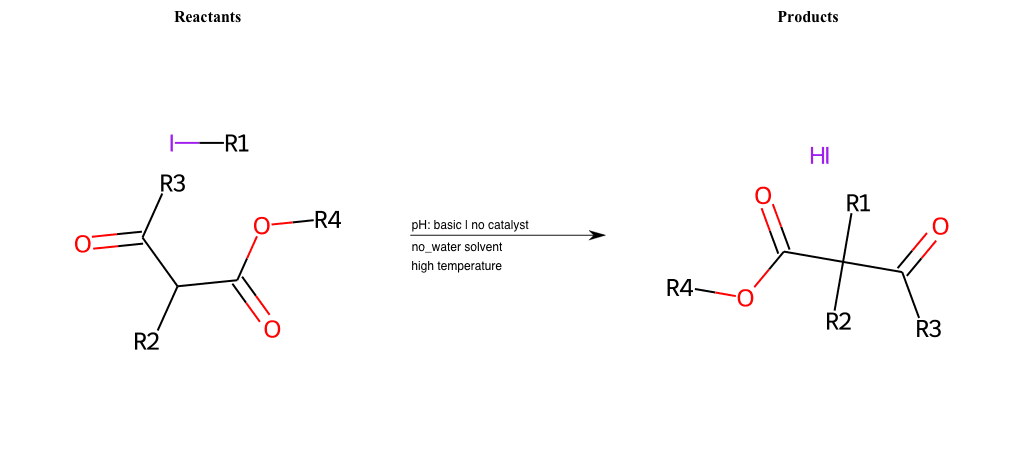
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Iodine-and-Nu-Hydroxyl
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
Condition to enforce:
R1 = A-Aliphatic-Carbon
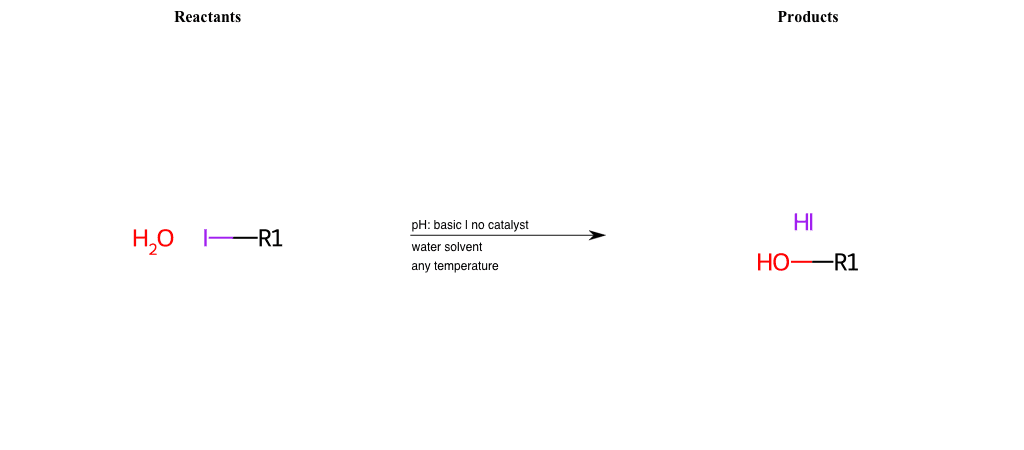
# Nucleophilic-Aliphatic-Substitution-Acidic-Lg-Nitrate-and-Nu-Hydroxyl
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Nitrate ester - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
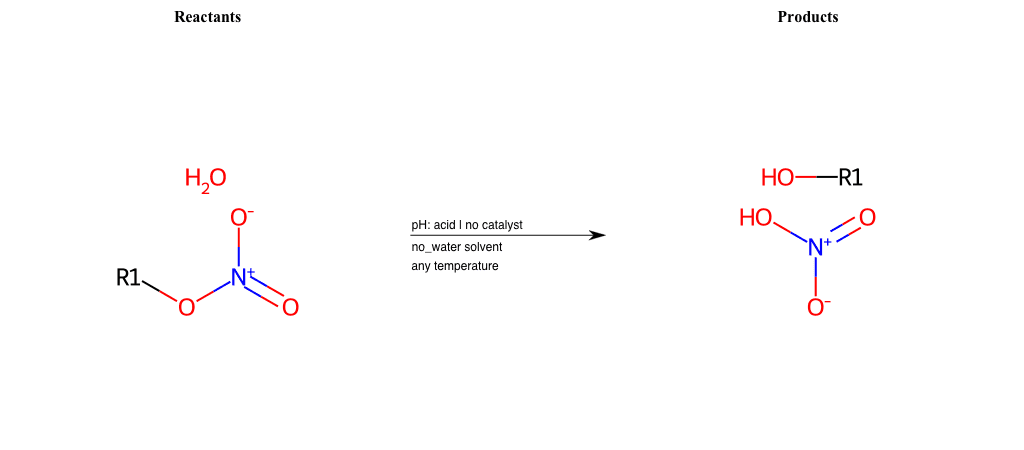
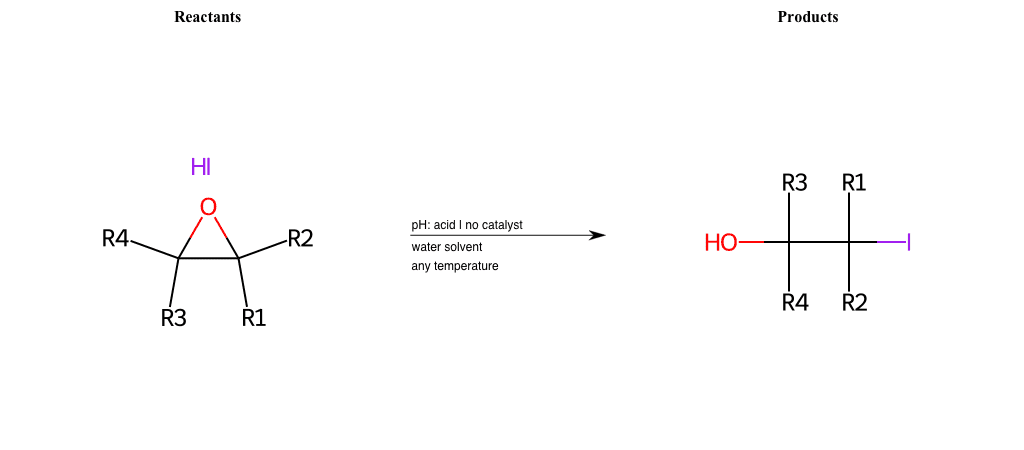
# Epoxide-Ring-Opening-Nu-Iodine-acid
References:
[0]
Epoxidation - Chemistry LibreTexts
[1]
Epoxides Ring-Opening Reactions - Chemistry Steps
Condition to enforce:
R1 = L-A, A-Aromatic-Carbon
R2 = L-A, A-Aromatic-Carbon
R3 = L-A, A-Aromatic-Carbon
R4 = L-A, A-Aromatic-Carbon
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Bromine-and-Nu-ONO
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Haloalkanes react with KNO2 to form alkyl nitrites while AgNO2 forms nitroalkanes as the
chief product.
Condition to enforce:
R1 = A-Aliphatic-Carbon
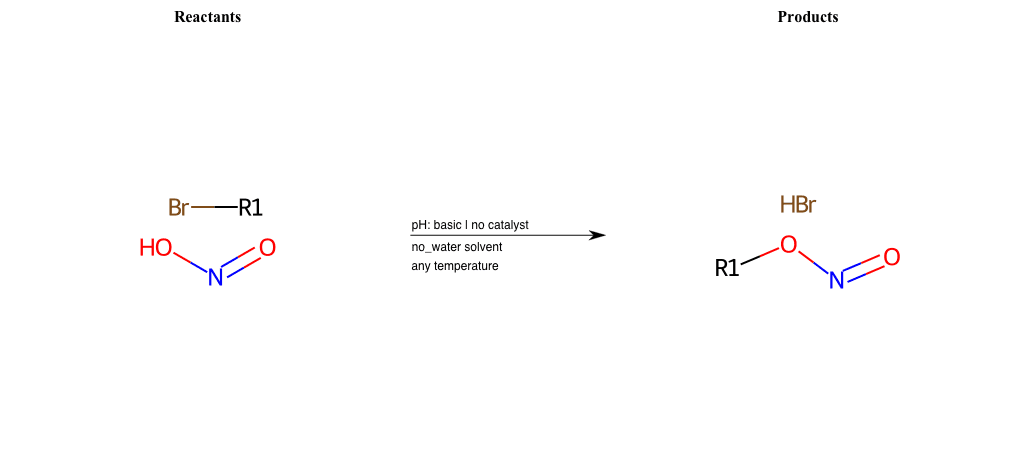
# Nucleophilic-Aliphatic-Substitution-Acidic-Lg-Alkoxide-and-Nu-Bromine
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Acidic cleavage of ethers (SN2) – Master Organic Chemistry
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = A-Aliphatic-Carbon
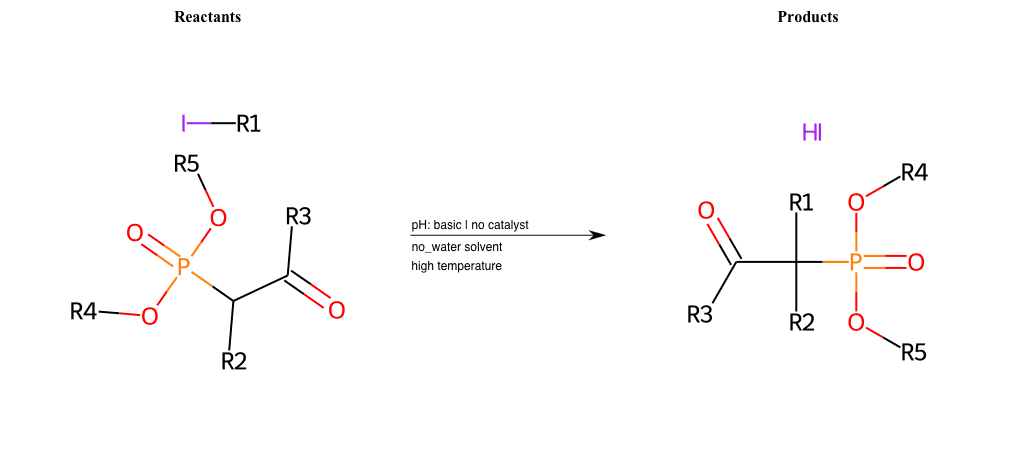
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carbonyl-and-EWG2-Phosphonate-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R5 = A-Aliphatic-Carbon, A-Aromatic-Carbon
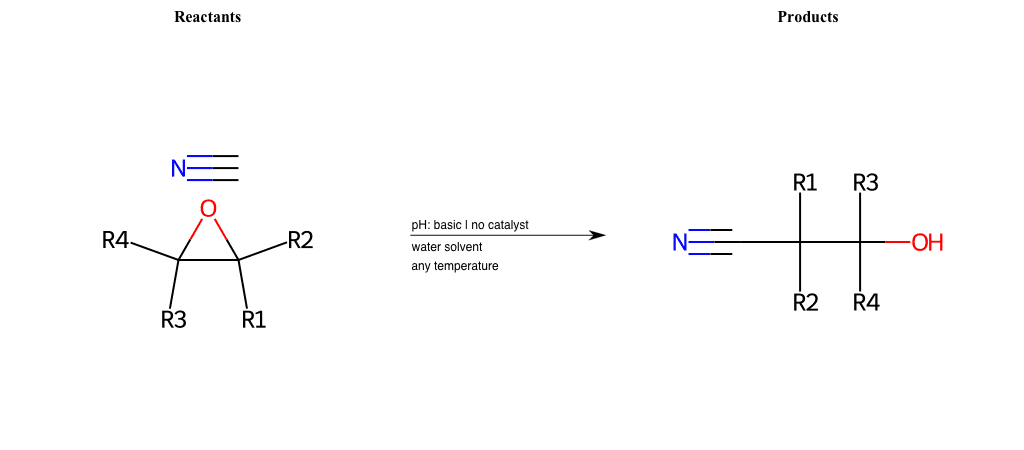
# Epoxide-Ring-Opening-Nu-Nitrile-basic
References:
[0]
Epoxidation - Chemistry LibreTexts
[1]
Epoxides Ring-Opening Reactions - Chemistry Steps
Condition to enforce:
R1 = L-A, A-Aromatic-Carbon
R2 = L-A, A-Aromatic-Carbon
R3 = L-A, A-Aromatic-Carbon
R4 = L-A, A-Aromatic-Carbon
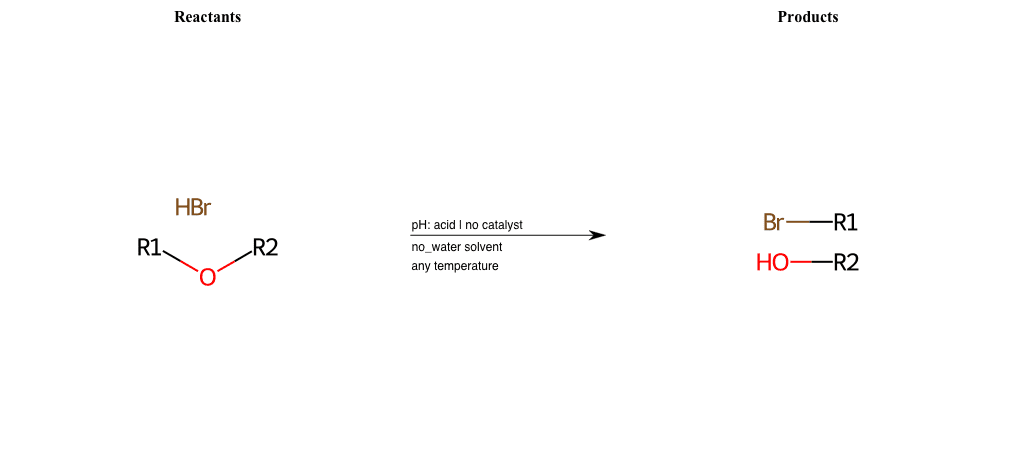
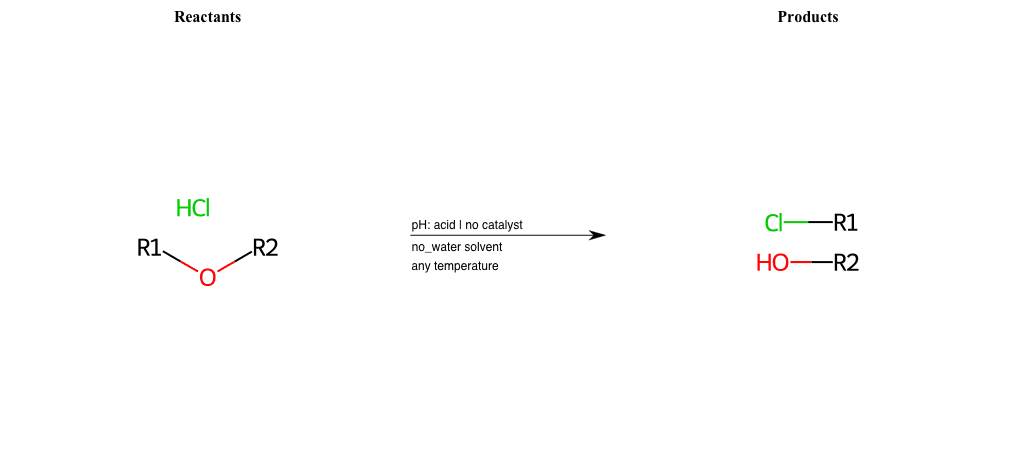
# Nucleophilic-Aliphatic-Substitution-Acidic-Lg-Alkoxide-and-Nu-Chlorine
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Acidic cleavage of ethers (SN2) – Master Organic Chemistry
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = A-Aliphatic-Carbon
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carbonyl-and-EWG2-Carbonyl-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R4 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
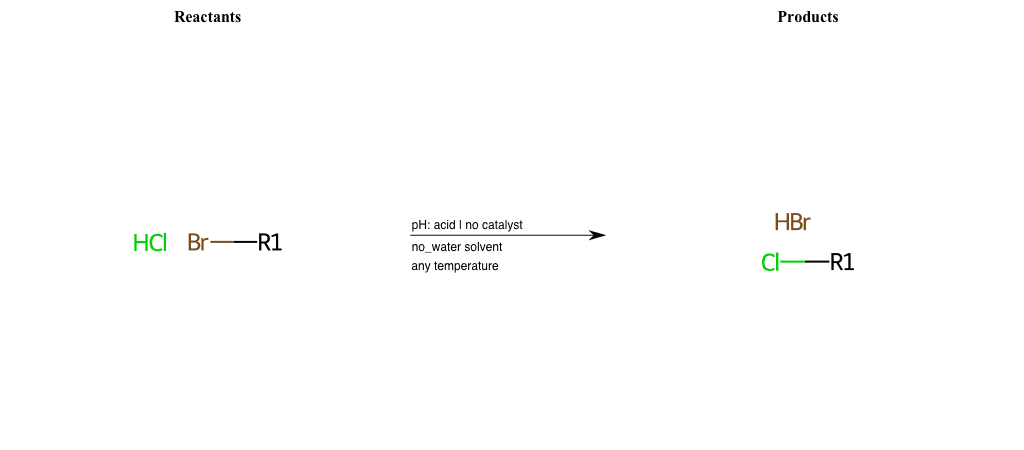
# Nucleophilic-Aliphatic-Substitution-Acidic-Lg-Bromine-and-Nu-Chlorine
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
Condition to enforce:
R1 = A-Aliphatic-Carbon
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carboxyl-and-EWG2-Nitrite-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Iodine-and-Nu-Carboxyl-H
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
carboxylic-acid-as-nucleophile
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
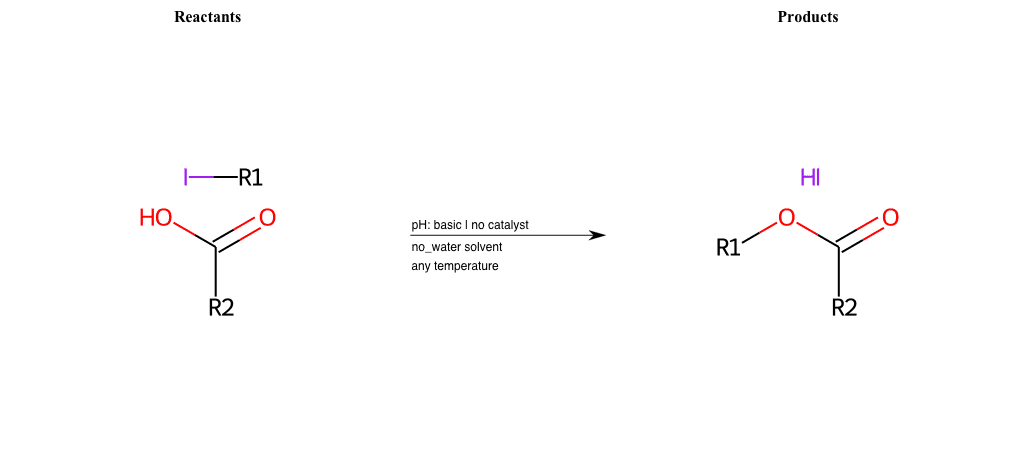
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carbonyl-and-EWG2-Nitrile-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
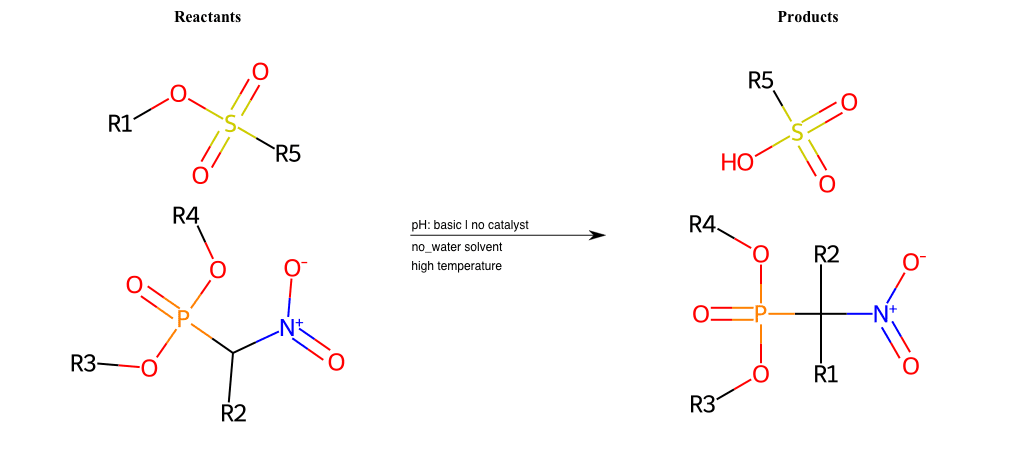
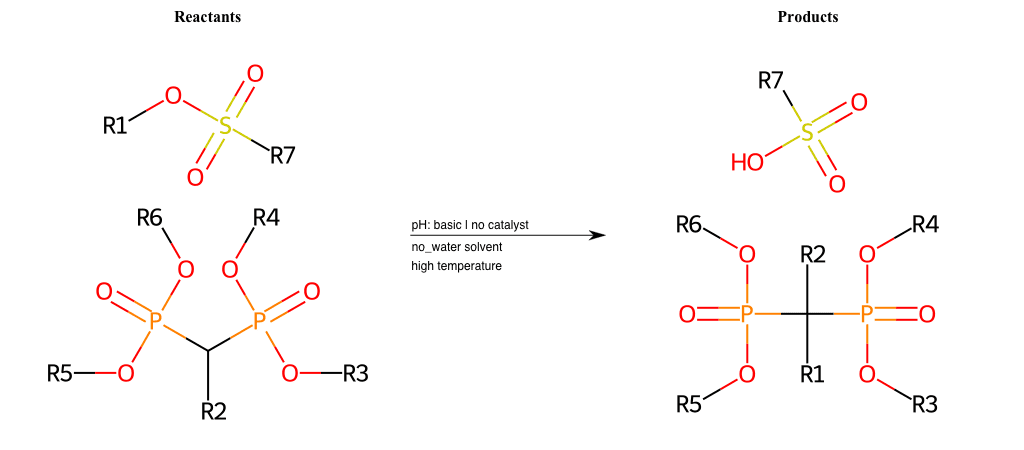
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Phosphonate-and-EWG2-Phosphonate-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R5 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R6 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R7 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Alkane-and-EWG2-Phosphonate-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R5 = A-Aliphatic-Carbon, A-Aromatic-Carbon
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Alkane-and-EWG2-Phosphonate-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R5 = A-Aliphatic-Carbon, A-Aromatic-Carbon
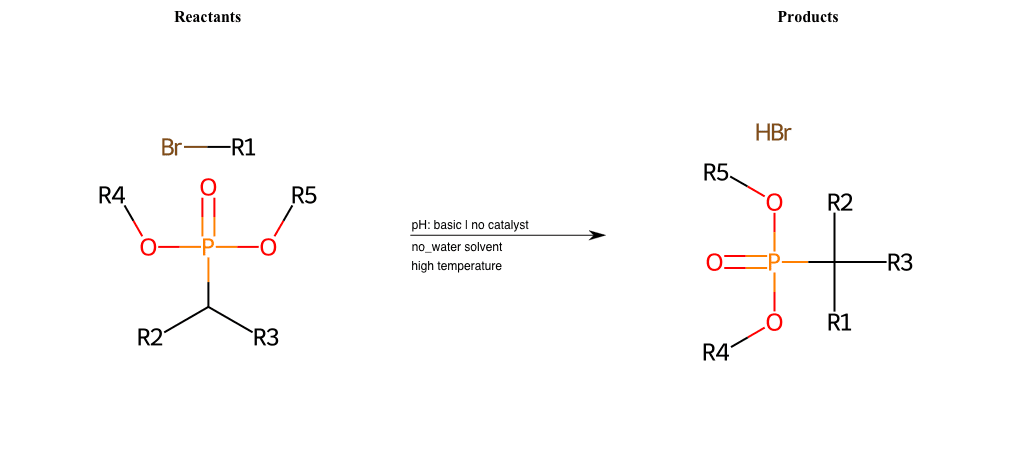
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carboxyl-and-EWG2-Phosphonate-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R5 = A-Aliphatic-Carbon, A-Aromatic-Carbon
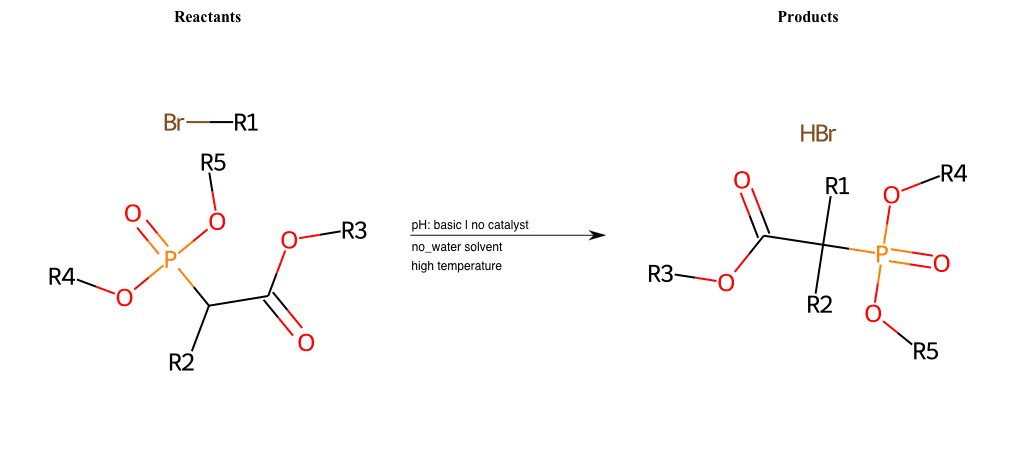
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carboxyl-and-EWG2-Nitrite-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
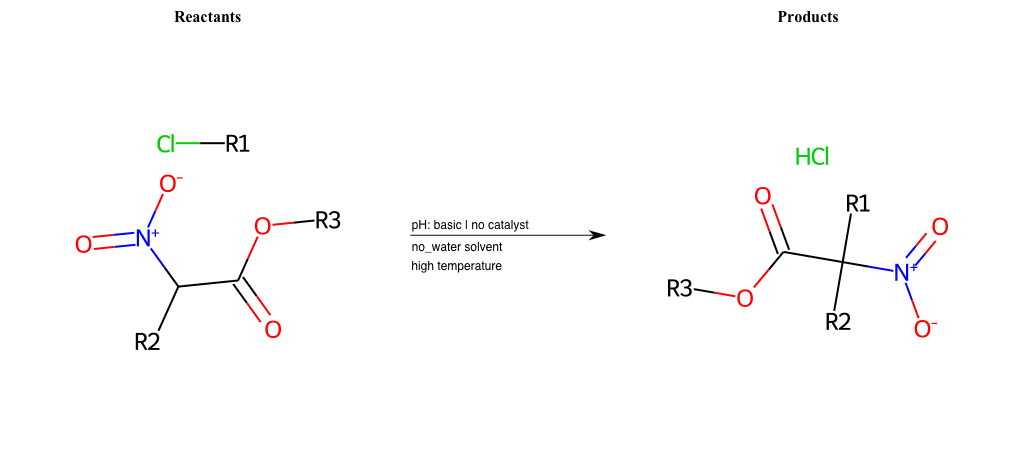
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Bromine-and-Nu-Nitrile
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Kolbe Nitrile Synthesis
[4]
Pelouze Synthesis
Condition to enforce:
R1 = A-Aliphatic-Carbon
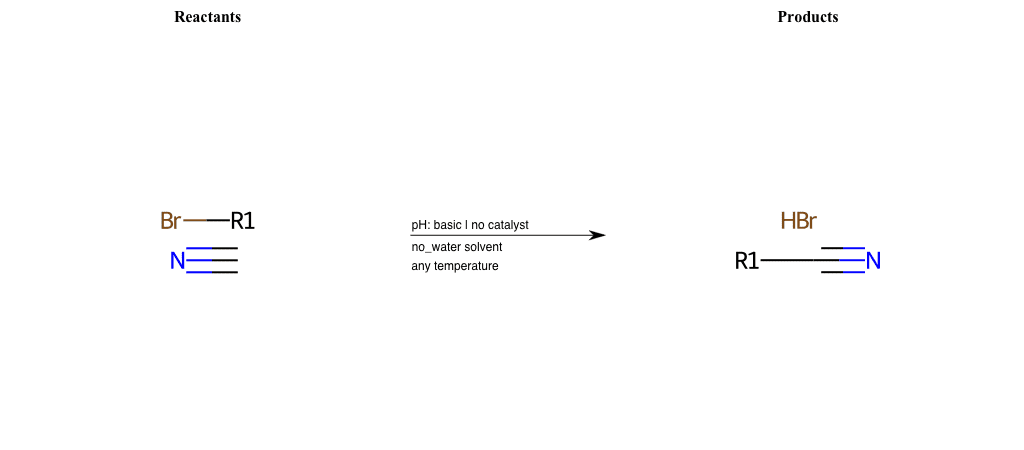
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carbonyl-and-EWG2-Nitrile-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
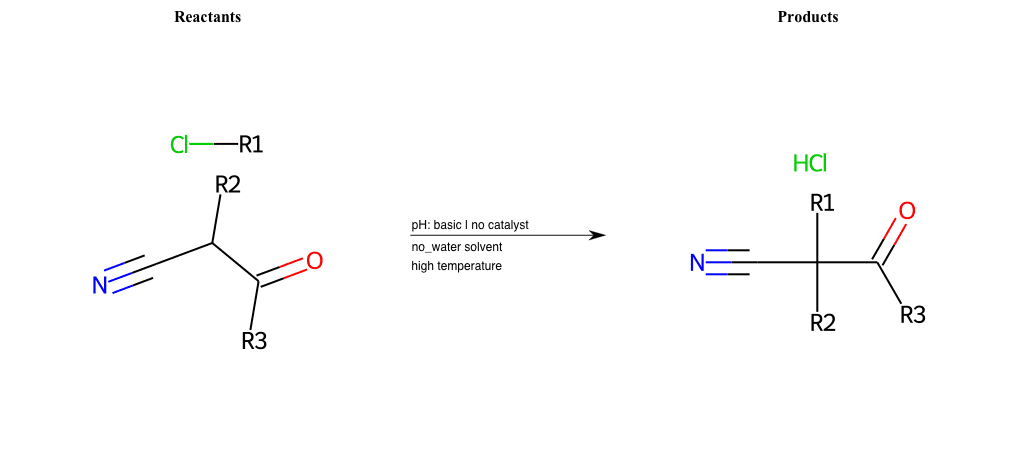
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Sulfonate-and-Nu-ONO
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Haloalkanes react with KNO2 to form alkyl nitrites while AgNO2 forms nitroalkanes as the
chief product.
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
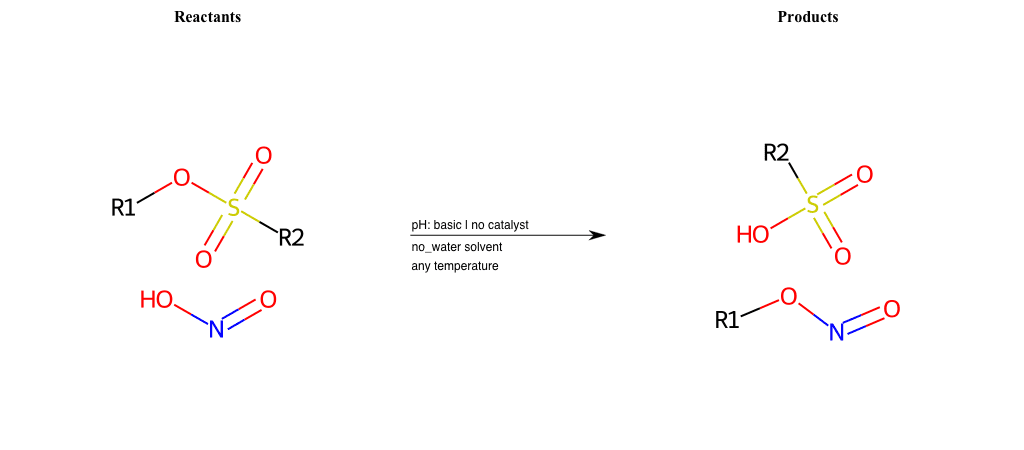
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Iodine-and-Nu-Nitrile
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Kolbe Nitrile Synthesis
[4]
Pelouze Synthesis
Condition to enforce:
R1 = A-Aliphatic-Carbon
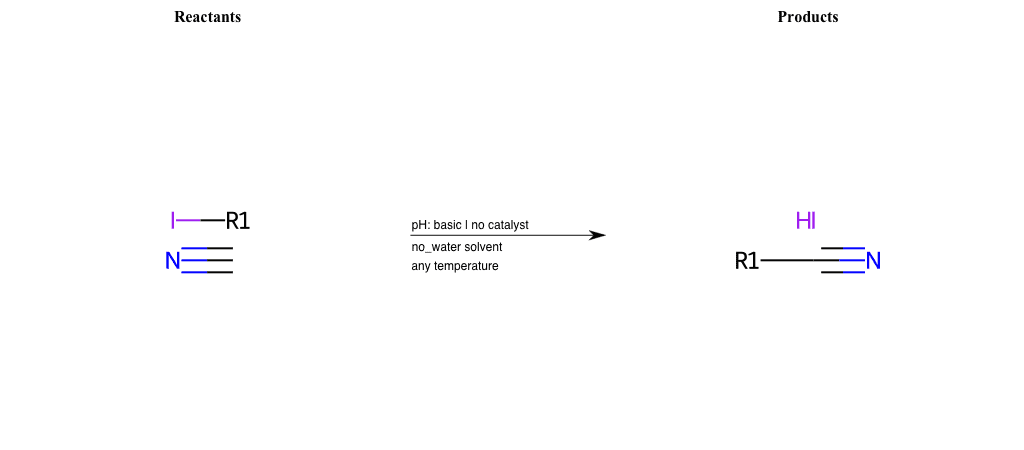
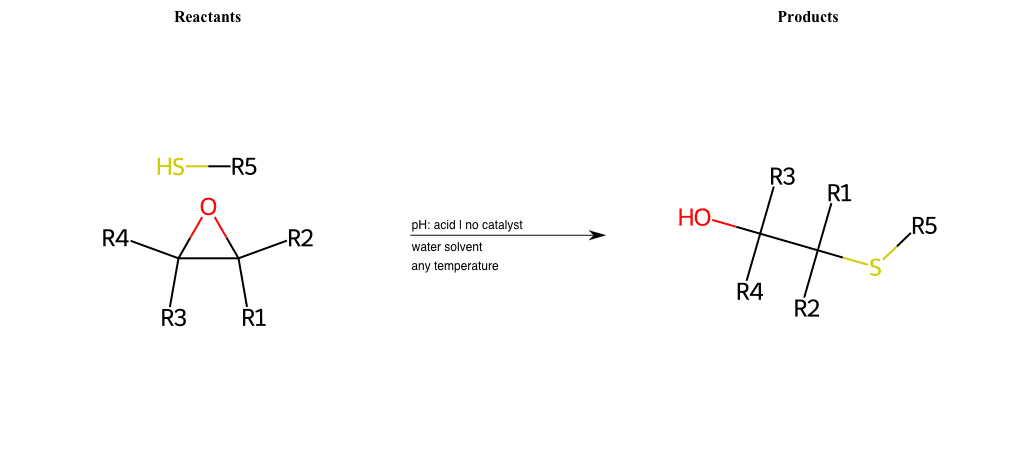
# Epoxide-Ring-Opening-Nu-Thiolate-basic
References:
[0]
Epoxidation - Chemistry LibreTexts
[1]
Epoxides Ring-Opening Reactions - Chemistry Steps
Condition to enforce:
R1 = L-A, A-Aromatic-Carbon
R2 = L-A, A-Aromatic-Carbon
R3 = L-A, A-Aromatic-Carbon
R4 = L-A, A-Aromatic-Carbon
R5 = A-Aliphatic-Carbon, A-Aromatic-Carbon
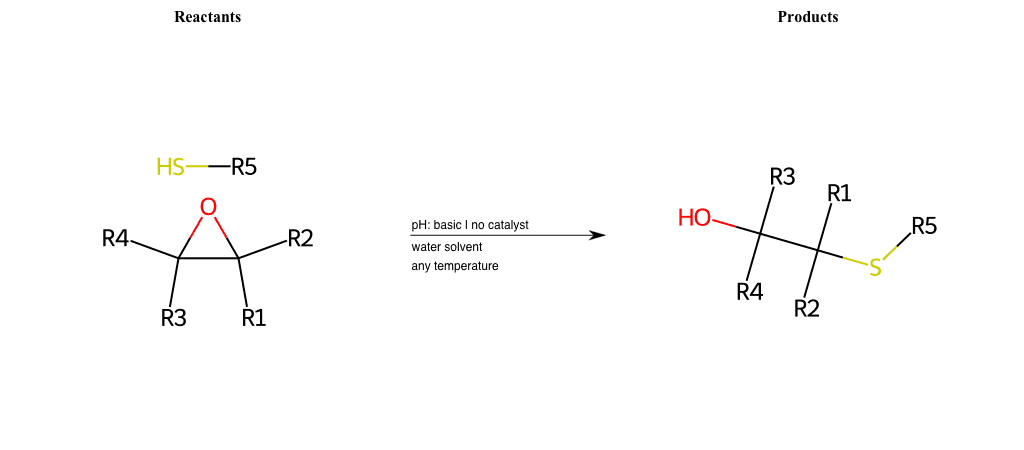
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Sulfonate-and-Nu-Carboxyl-H
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
carboxylic-acid-as-nucleophile
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R3 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
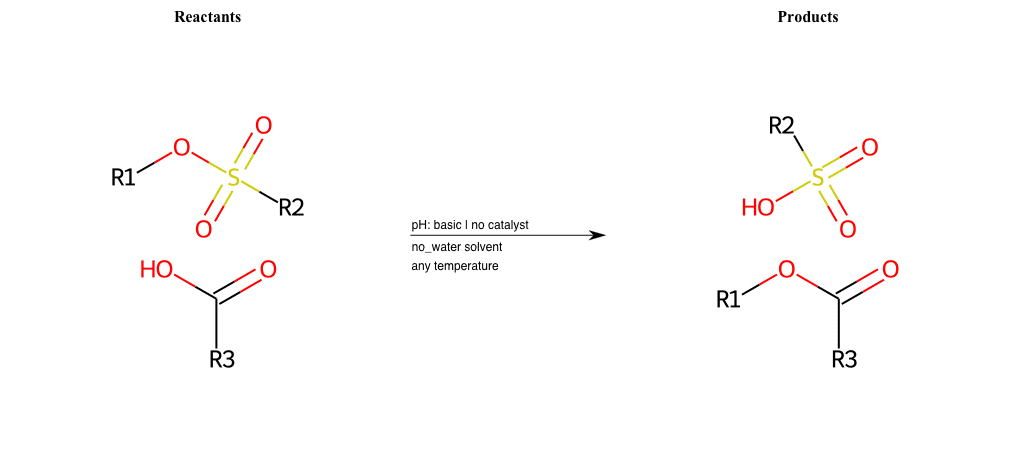
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carbonyl-and-EWG2-Carboxyl-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
R5 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
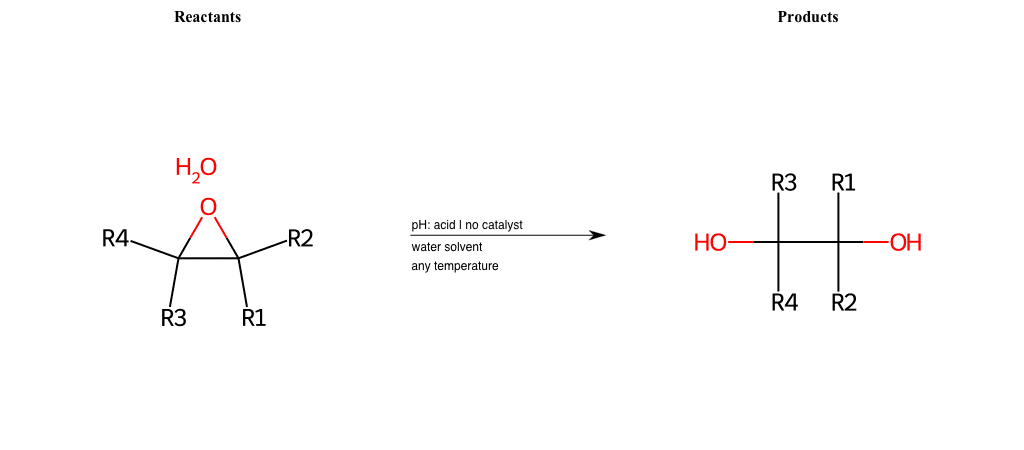
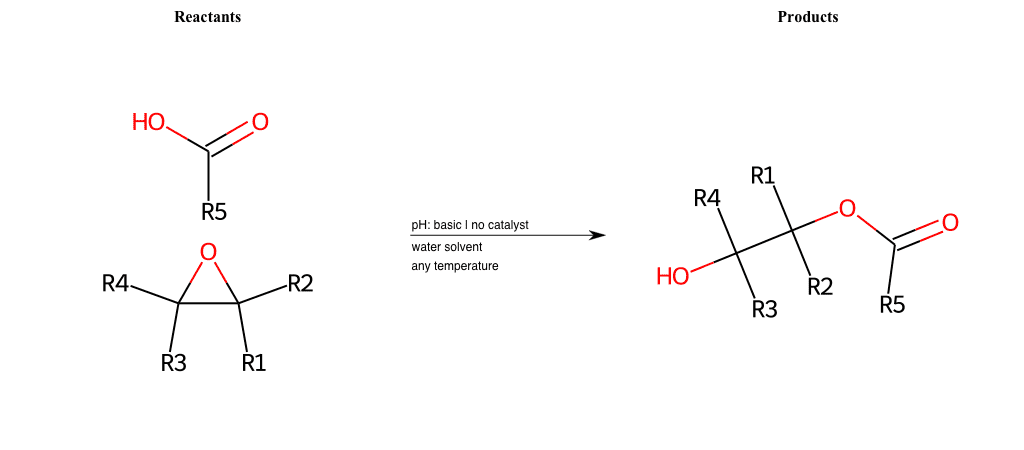
# Epoxide-Ring-Opening-Nu-Hydroxyl-acid
References:
[0]
Epoxidation - Chemistry LibreTexts
[1]
Epoxides Ring-Opening Reactions - Chemistry Steps
Condition to enforce:
R1 = L-A, A-Aromatic-Carbon
R2 = L-A, A-Aromatic-Carbon
R3 = L-A, A-Aromatic-Carbon
R4 = L-A, A-Aromatic-Carbon
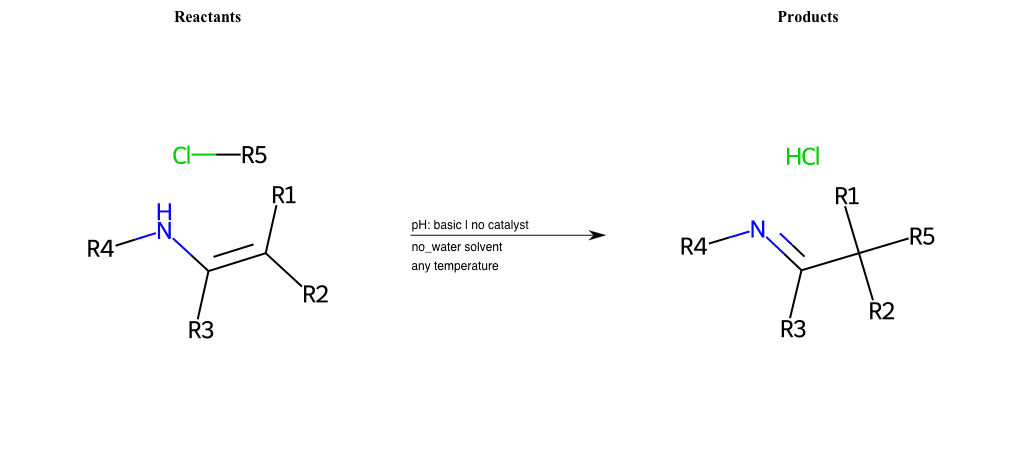
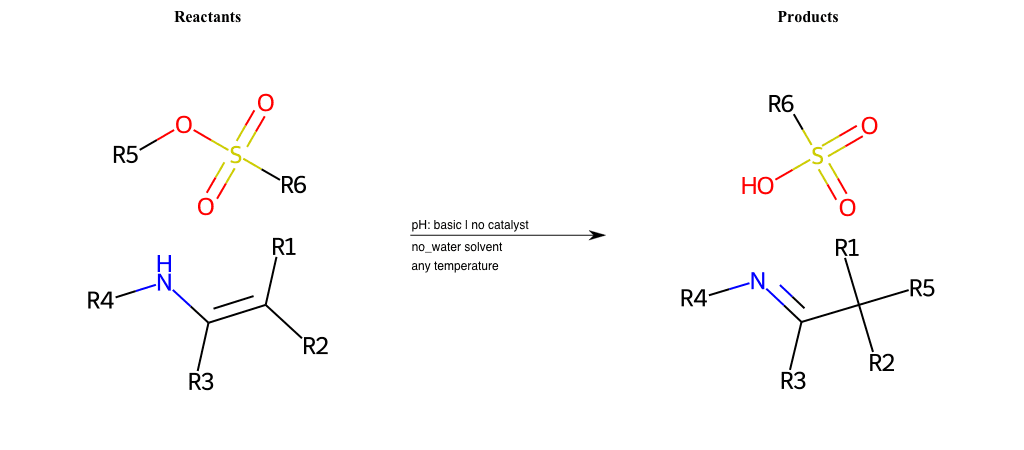
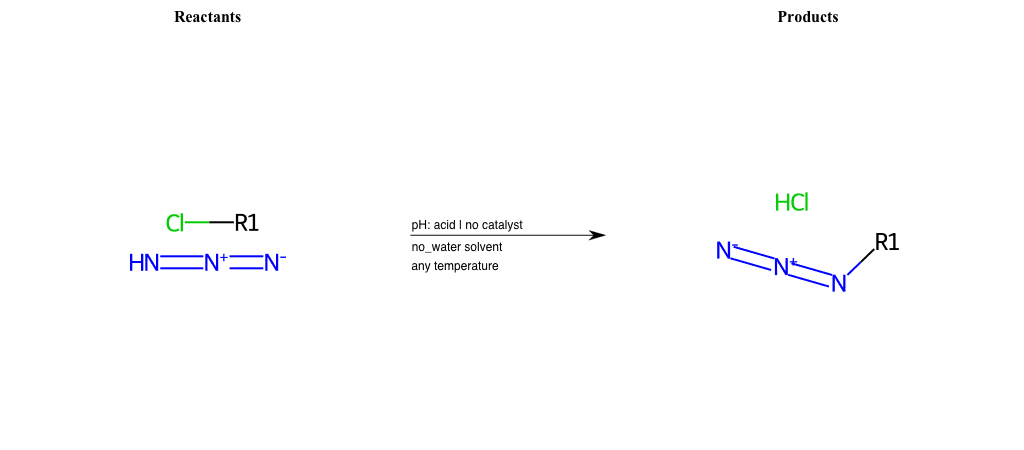
# Nucleophilic-Substitution-Enamine-Lg-Chlorine
References:
[0]
Enamine - Wikipedia
Condition to enforce:
R1 = H, A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon
R4 = H, A-Aliphatic-Carbon
R5 = A-Aliphatic-Carbon
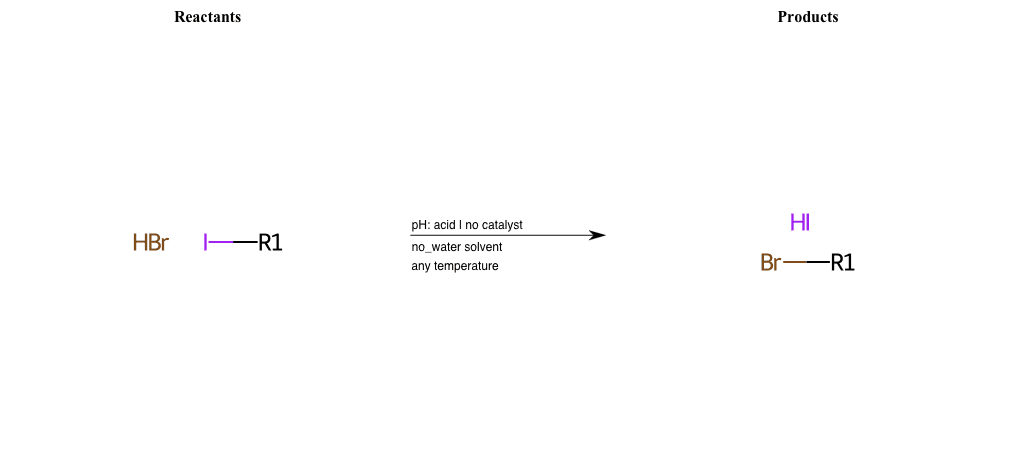
# Nucleophilic-Aliphatic-Substitution-Acidic-Lg-Iodine-and-Nu-Bromine
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
Condition to enforce:
R1 = A-Aliphatic-Carbon
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carbonyl-and-EWG2-Nitrite-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Sulfonate-and-Nu-O-Carbamate
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
R4 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Nitrite-and-EWG2-Nitrite-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Chlorine-and-Nu-Azide
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Reactions of Azides - Substitution, Reduction, Rearrangements, and More
Condition to enforce:
R1 = A-Aliphatic-Carbon
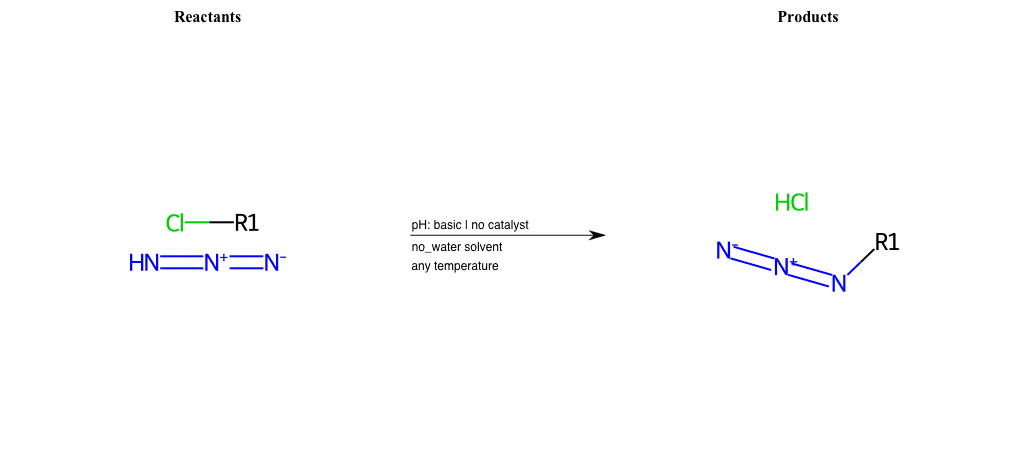
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Iodine-and-Nu-Azide
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Reactions of Azides - Substitution, Reduction, Rearrangements, and More
Condition to enforce:
R1 = A-Aliphatic-Carbon
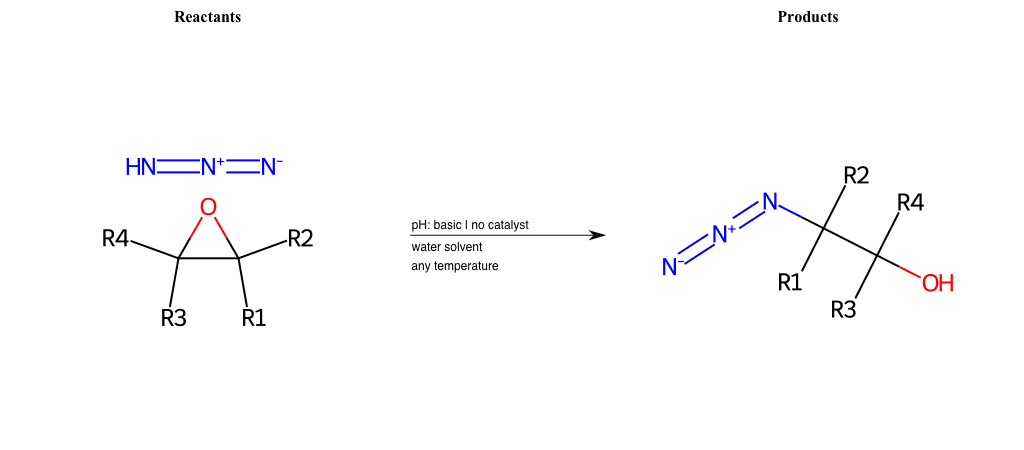
# Epoxide-Ring-Opening-Nu-Azide-basic
References:
[0]
Epoxidation - Chemistry LibreTexts
[1]
Epoxides Ring-Opening Reactions - Chemistry Steps
Condition to enforce:
R1 = L-A, A-Aromatic-Carbon
R2 = L-A, A-Aromatic-Carbon
R3 = L-A, A-Aromatic-Carbon
R4 = L-A, A-Aromatic-Carbon
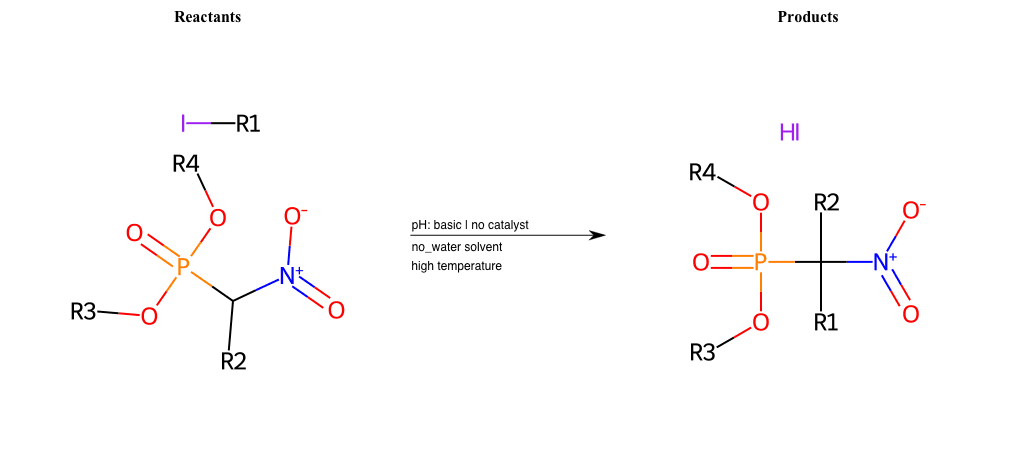
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Nitrite-and-EWG2-Phosphonate-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Alkane-and-EWG2-Carboxyl-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
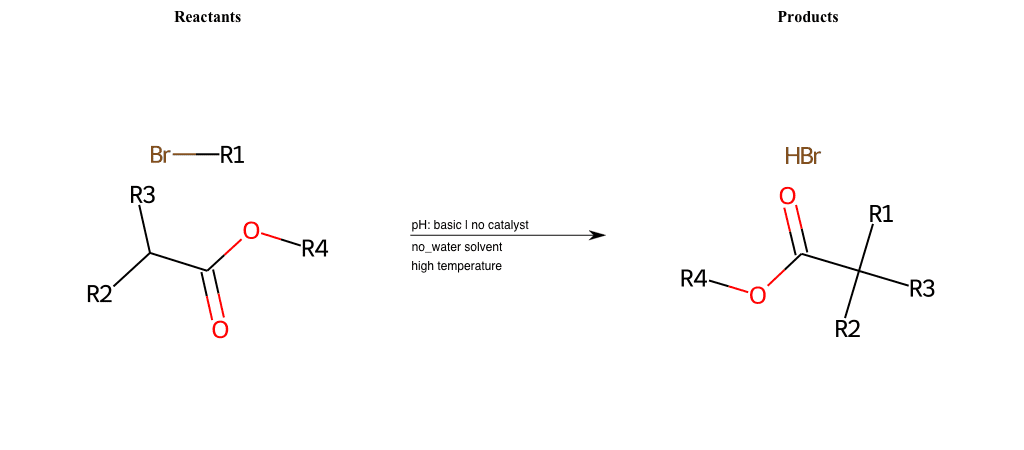
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Alkane-and-EWG2-Nitrite-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon
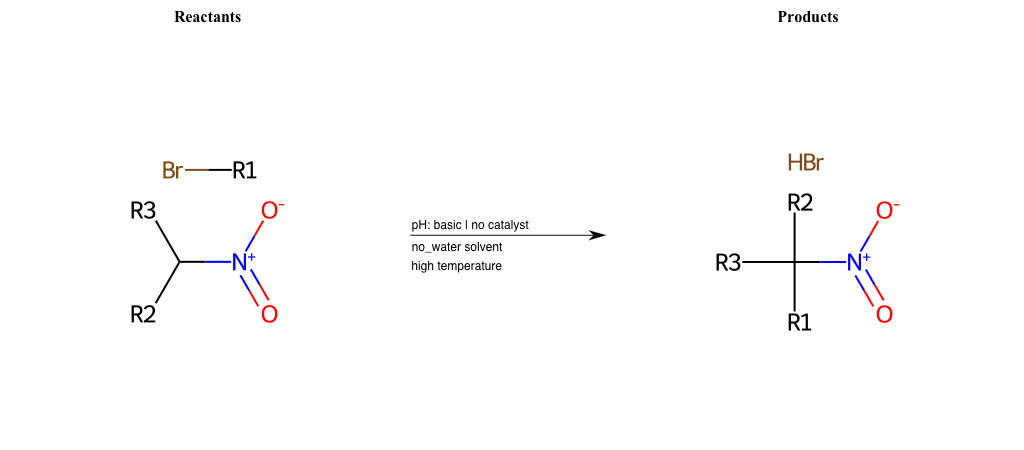
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Chlorine-and-Nu-Alkoxide
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Williamson ether synthesis - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = A-Aliphatic-Carbon, A-Aromatic-Carbon
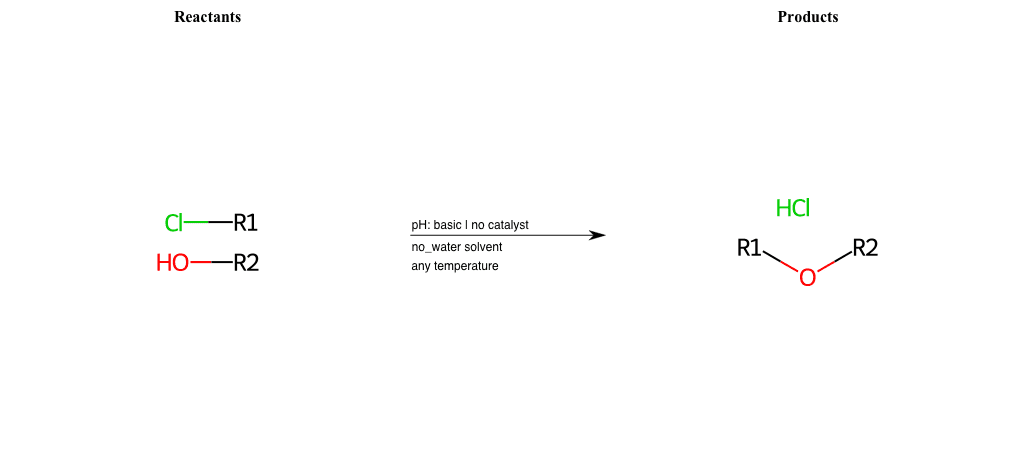
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Chlorine-and-Nu-Nitrate
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
Condition to enforce:
R1 = A-Aliphatic-Carbon
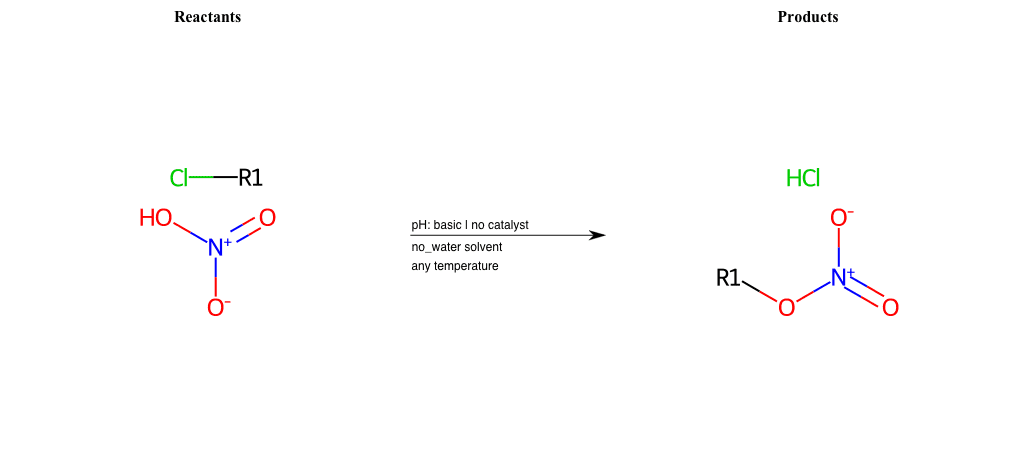
# Epoxide-Ring-Opening-Nu-Azide-acid
References:
[0]
Epoxidation - Chemistry LibreTexts
[1]
Epoxides Ring-Opening Reactions - Chemistry Steps
Condition to enforce:
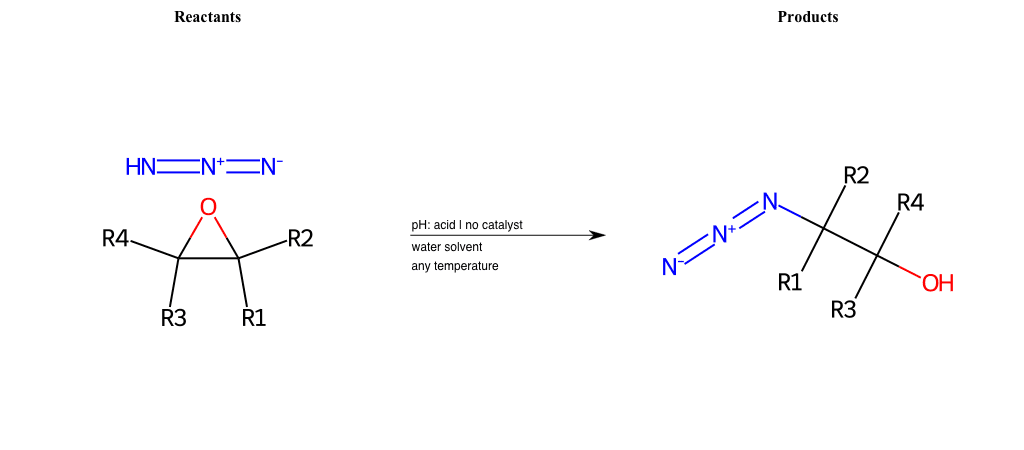
R1 = L-A, A-Aromatic-Carbon
R2 = L-A, A-Aromatic-Carbon
R3 = L-A, A-Aromatic-Carbon
R4 = L-A, A-Aromatic-Carbon
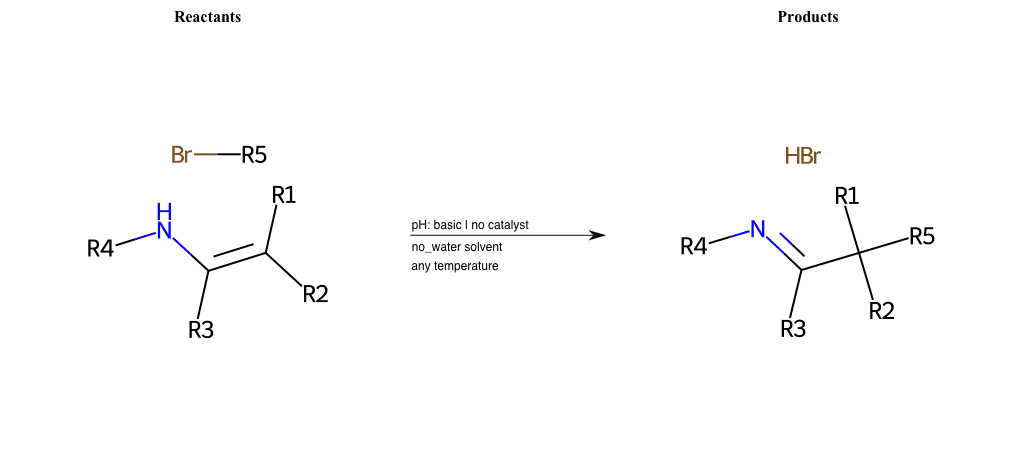
# Nucleophilic-Substitution-Enamine-Lg-Sulfonate
References:
[0]
Enamine - Wikipedia
Condition to enforce:
R1 = H, A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon
R4 = H, A-Aliphatic-Carbon
R5 = A-Aliphatic-Carbon
R6 = H, A-Aliphatic-Carbon
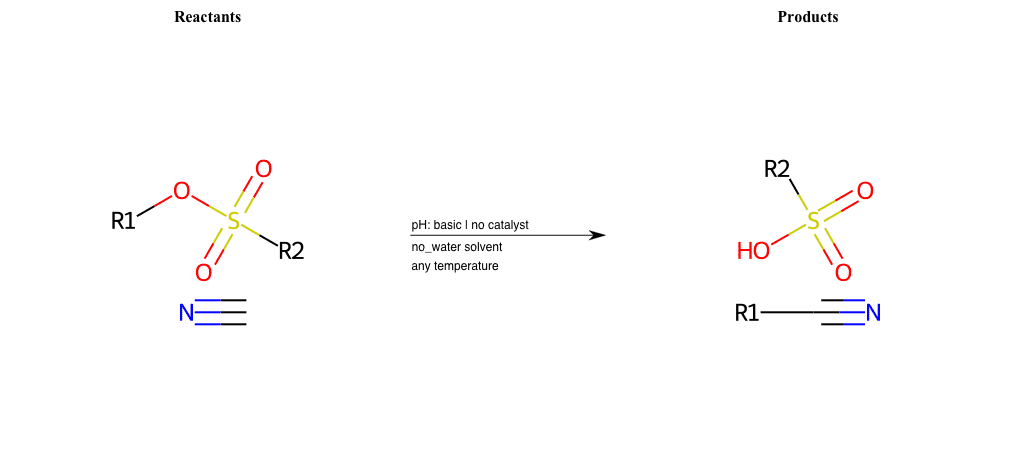
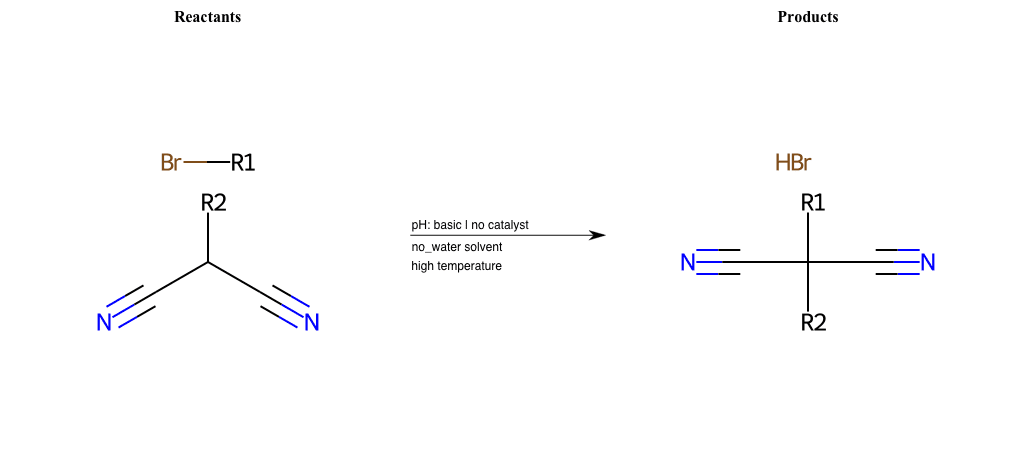
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Sulfonate-and-Nu-Nitrile
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Kolbe Nitrile Synthesis
[4]
Pelouze Synthesis
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Bromine-and-Nu-O-Carbamate
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Nitrile-and-EWG2-Phosphonate-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon
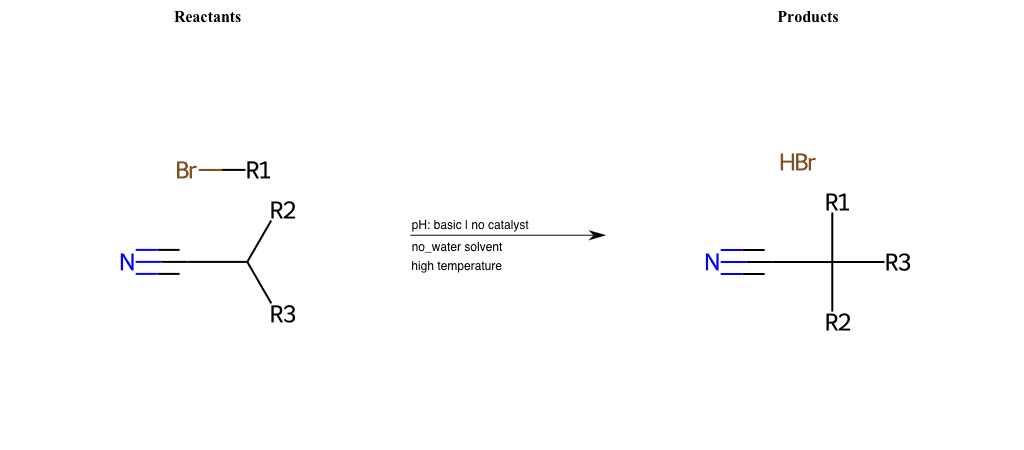
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Alkane-and-EWG2-Nitrile-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Chlorine-and-Nu-Amino
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Alkylation of Amines (Sucks!) – Master Organic Chemistry
[4]
9.4. Reaction of RX with NH3 and amines | Organic Chemistry 1: An open textbook
[5]
Amines as Nucleophiles - Chemistry LibreTexts
[6]
Gabriel Phthalimide Synthesis Mechanism - Explanation and Examples
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
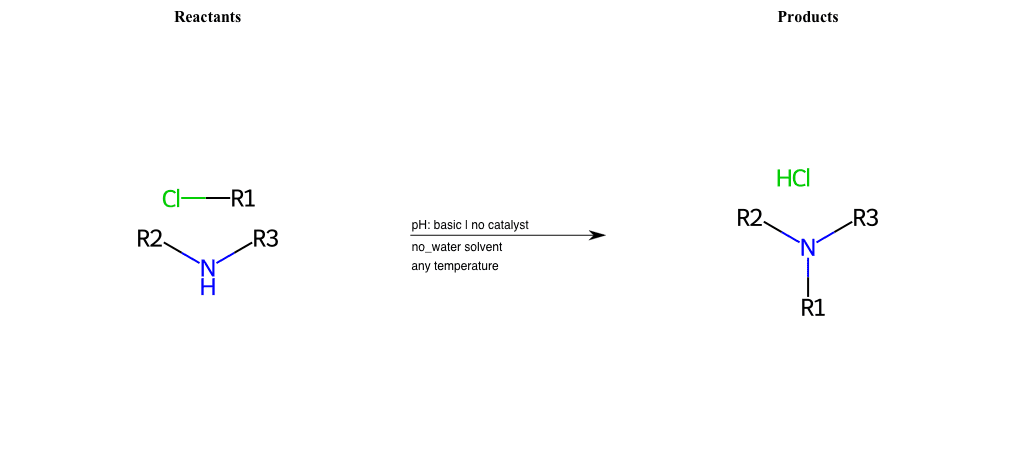
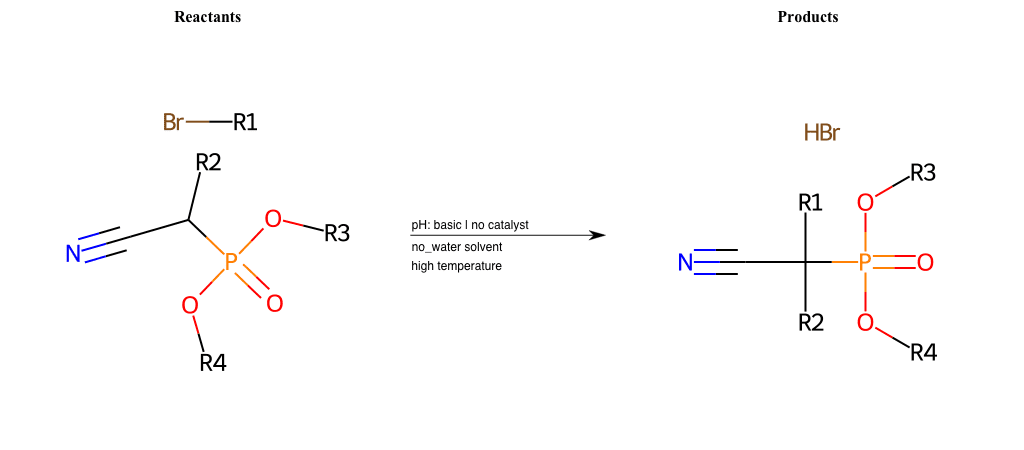
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carboxyl-and-EWG2-Nitrile-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
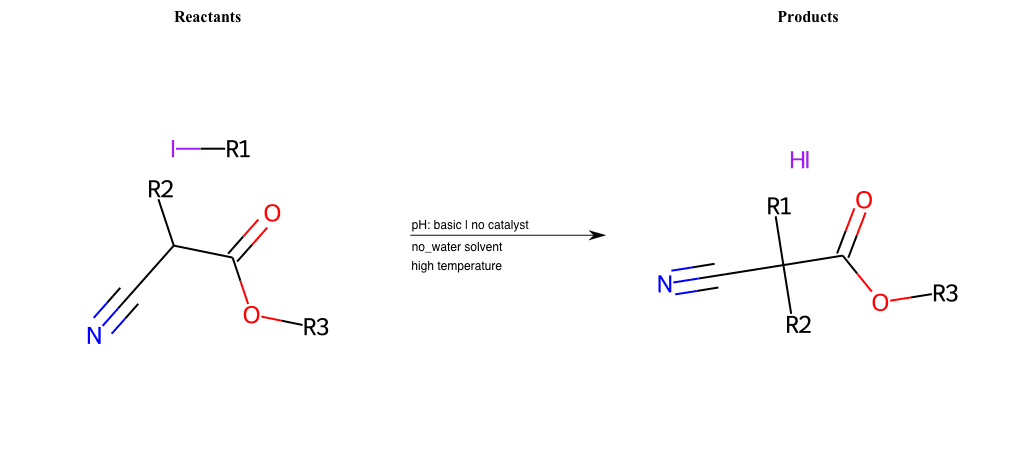
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carbonyl-and-EWG2-Nitrite-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
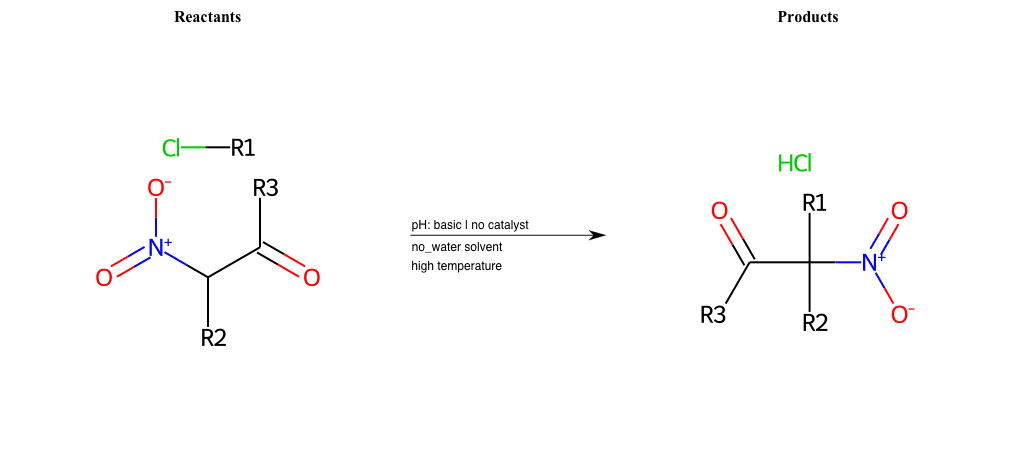
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carbonyl-and-EWG2-Phosphonate-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R5 = A-Aliphatic-Carbon, A-Aromatic-Carbon
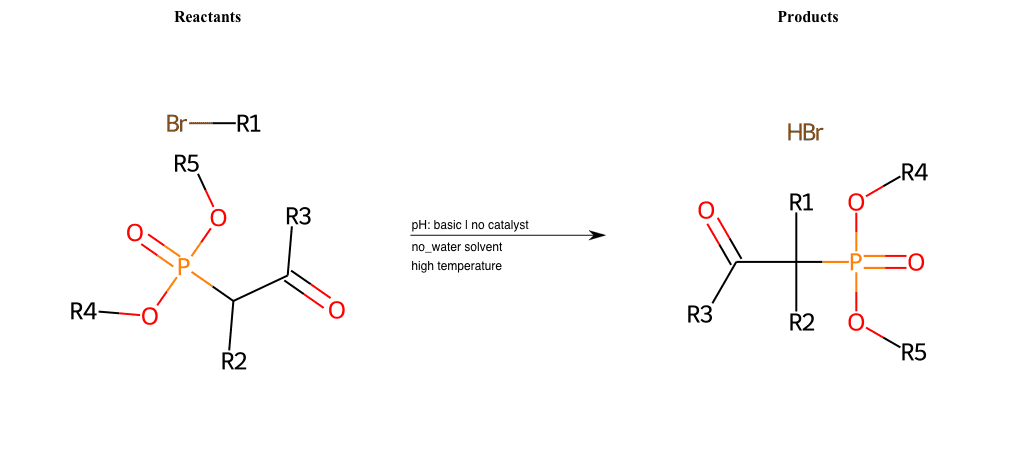
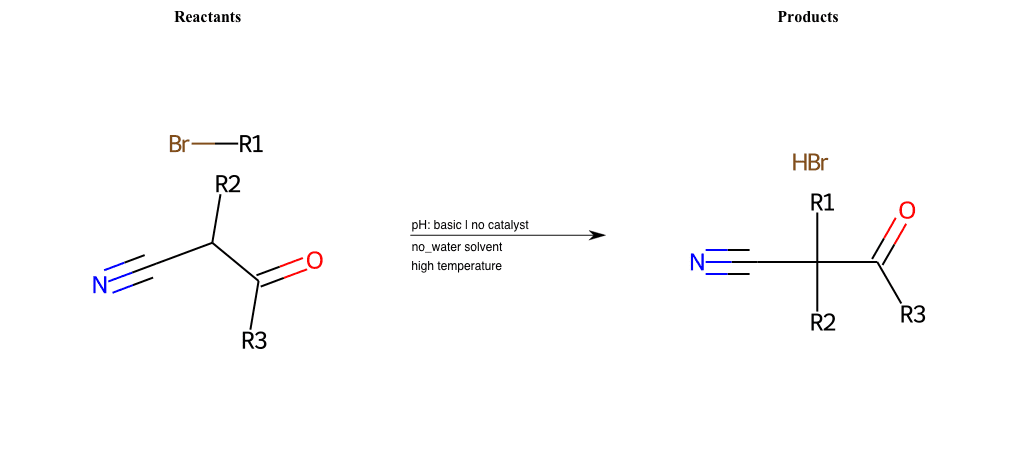
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carbonyl-and-EWG2-Nitrile-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
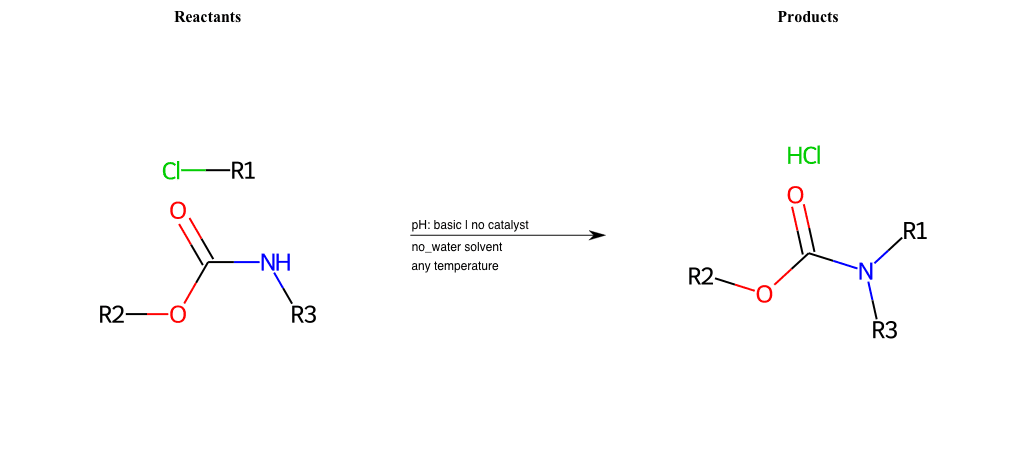
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Chlorine-and-Nu-N-Carbamate
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
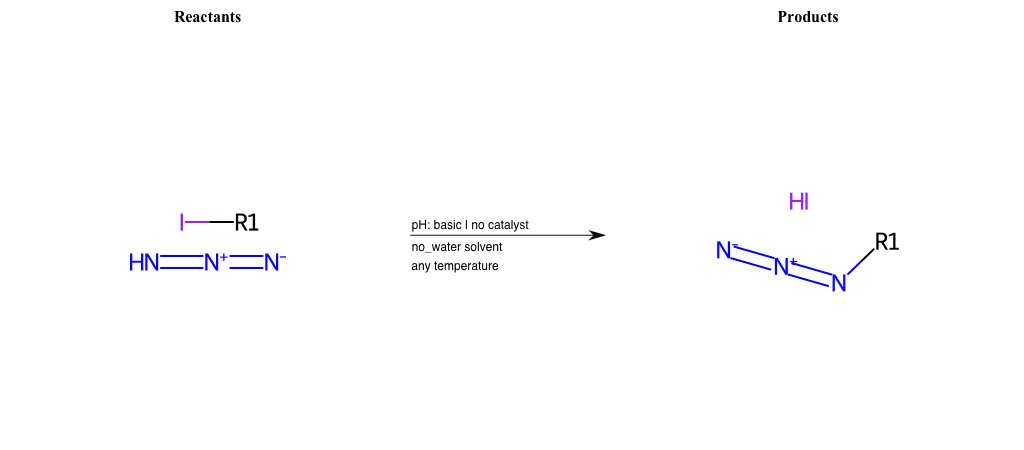
# Nucleophilic-Aliphatic-Substitution-Acidic-Lg-Iodine-and-Nu-Azide
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Reactions of Azides - Substitution, Reduction, Rearrangements, and More
Condition to enforce:
R1 = A-Aliphatic-Carbon
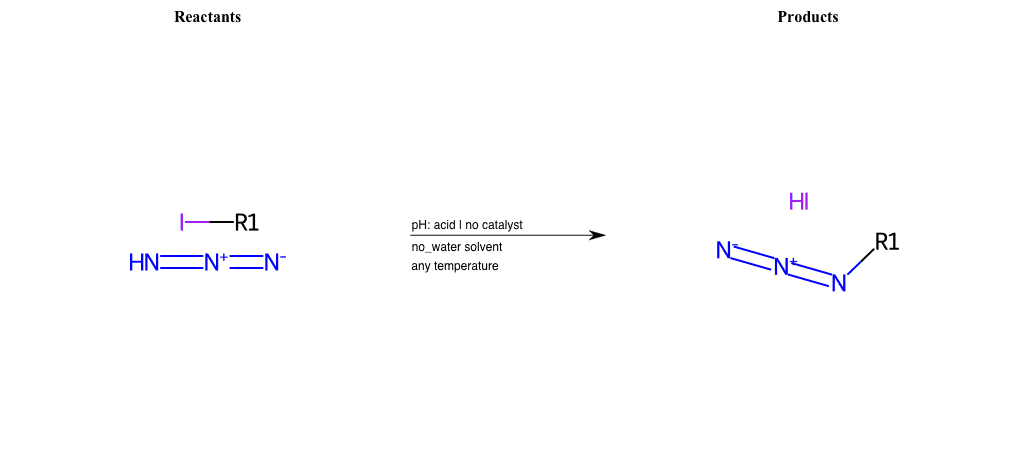
# Nucleophilic-Aliphatic-Substitution-Acidic-Lg-Sulfonate-and-Nu-Azide
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Reactions of Azides - Substitution, Reduction, Rearrangements, and More
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
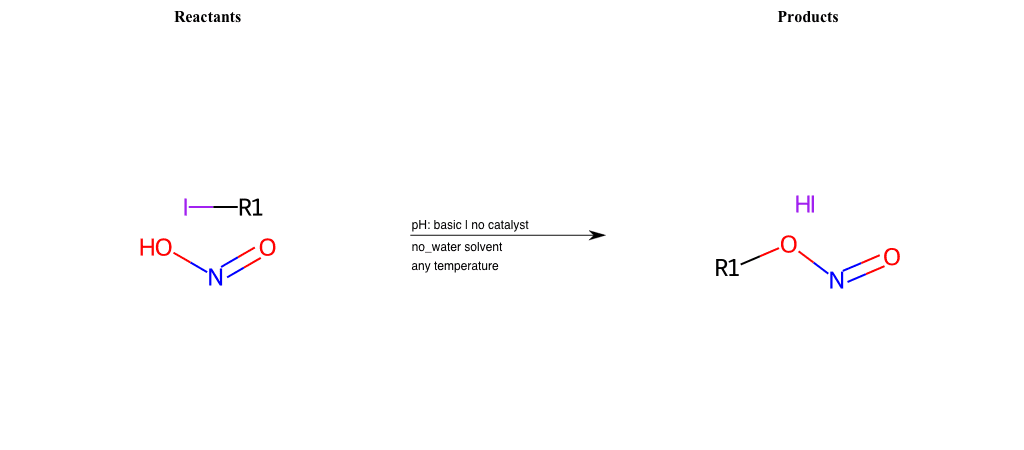
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Iodine-and-Nu-ONO
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Haloalkanes react with KNO2 to form alkyl nitrites while AgNO2 forms nitroalkanes as the
chief product.
Condition to enforce:
R1 = A-Aliphatic-Carbon
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Iodine-and-Nu-Amino
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Alkylation of Amines (Sucks!) – Master Organic Chemistry
[4]
9.4. Reaction of RX with NH3 and amines | Organic Chemistry 1: An open textbook
[5]
Amines as Nucleophiles - Chemistry LibreTexts
[6]
Gabriel Phthalimide Synthesis Mechanism - Explanation and Examples
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
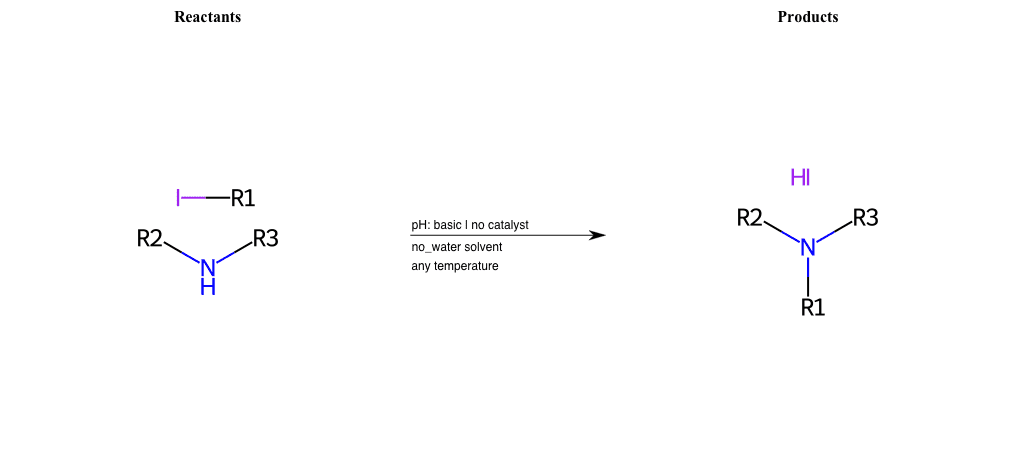
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Sulfonate-and-Nu-Thiolate
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R3 = A-Aliphatic-Carbon, A-Aromatic-Carbon
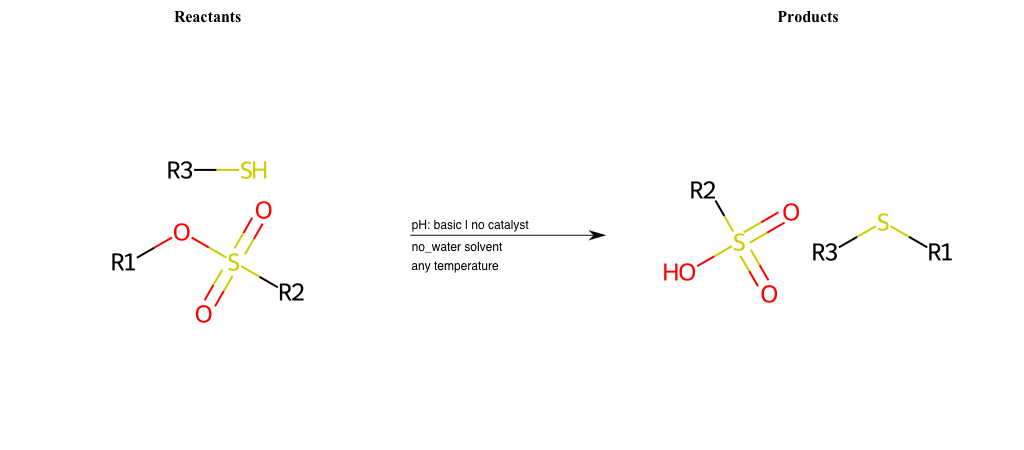
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Bromine-and-Nu-Alkoxide
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Williamson ether synthesis - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = A-Aliphatic-Carbon, A-Aromatic-Carbon
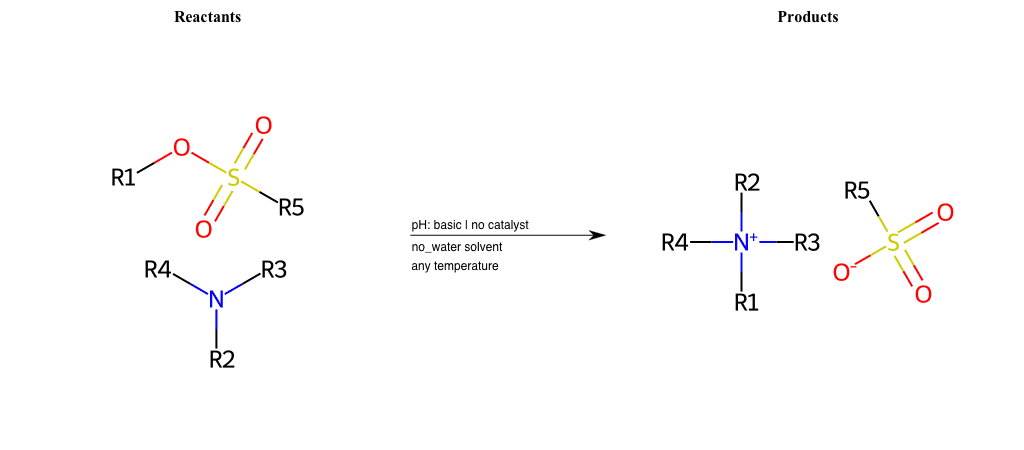
# Nucleophilic-Aliphatic-Substitution-Tertiary-Amine-Lg-Sulfonate
References:
[0]
Alkylation of Amines (Sucks!) – Master Organic Chemistry
[1]
9.4. Reaction of RX with NH3 and amines | Organic Chemistry 1: An open textbook
[2]
Amines as Nucleophiles - Chemistry LibreTexts
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R3 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R5 = A-Aliphatic-Carbon, A-Aromatic-Carbon
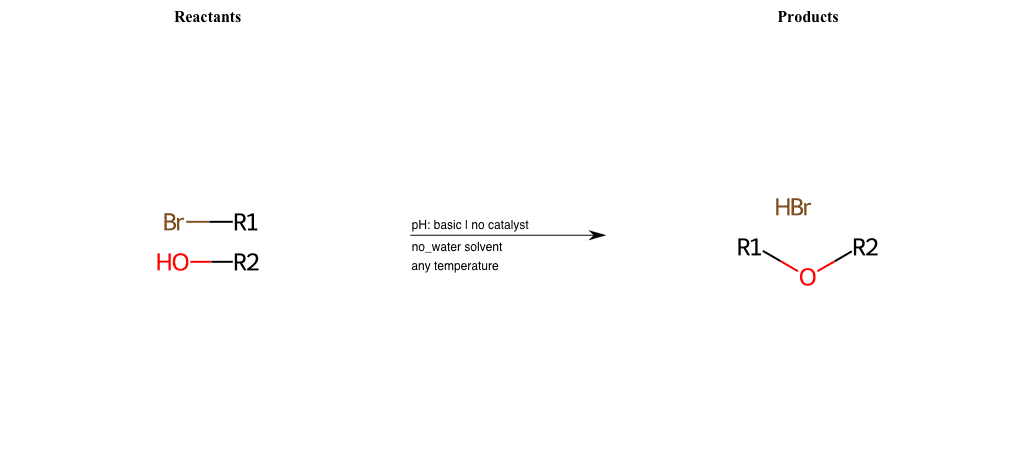
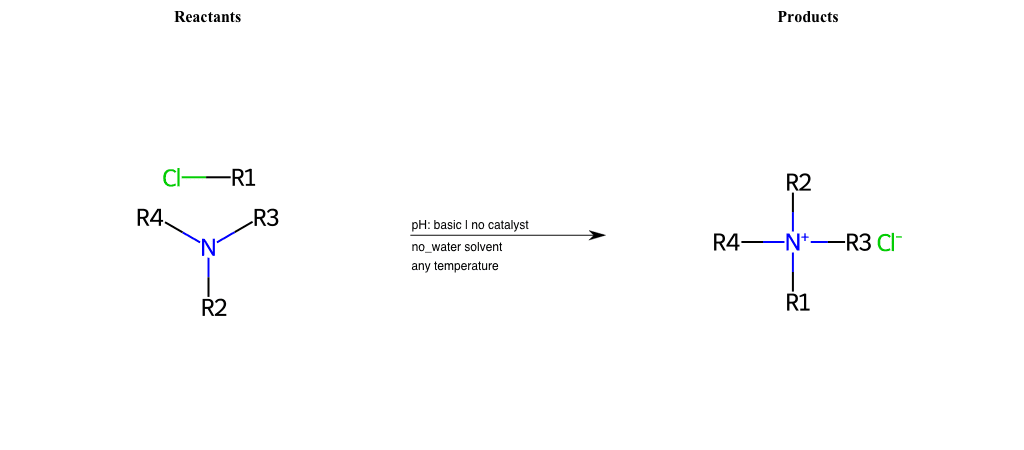
# Nucleophilic-Aliphatic-Substitution-Tertiary-Amine-Lg-Chlorine
References:
[0]
Alkylation of Amines (Sucks!) – Master Organic Chemistry
[1]
9.4. Reaction of RX with NH3 and amines | Organic Chemistry 1: An open textbook
[2]
Amines as Nucleophiles - Chemistry LibreTexts
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R3 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carboxyl-and-EWG2-Nitrile-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
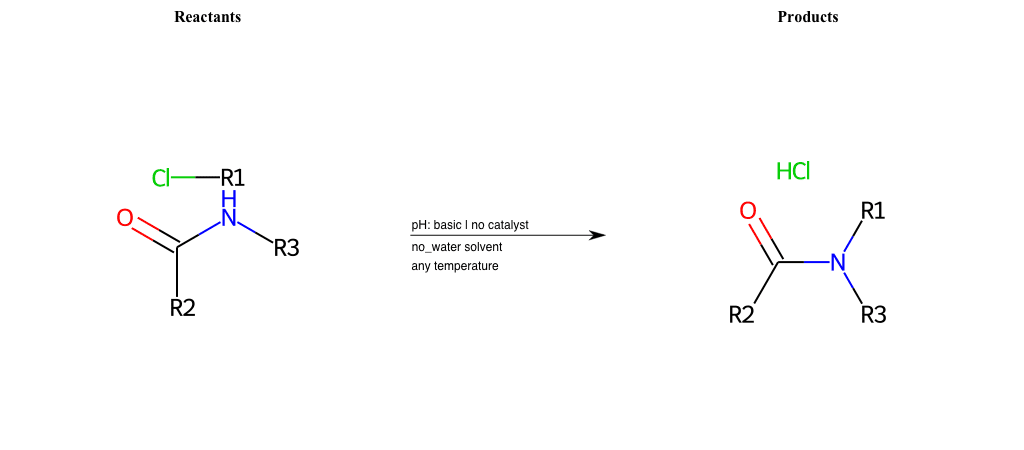
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Chlorine-and-Nu-N-Amide
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
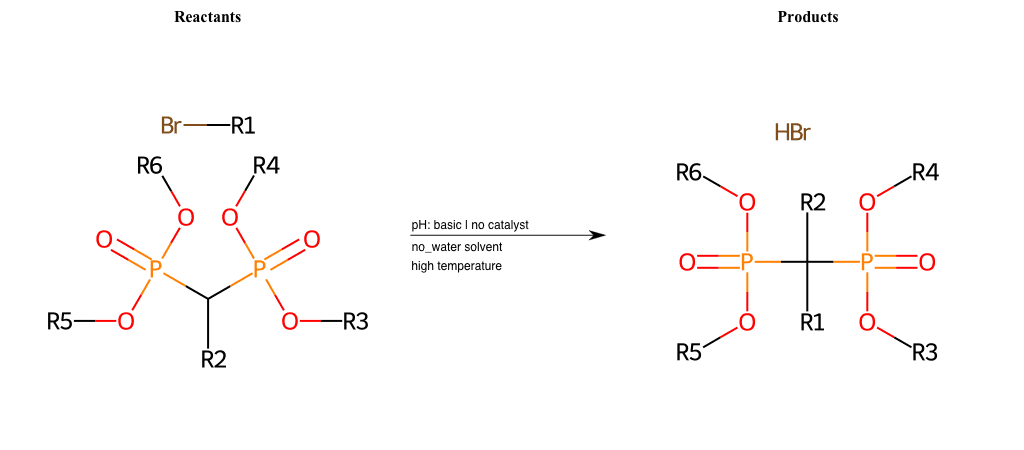
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Phosphonate-and-EWG2-Phosphonate-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R5 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R6 = A-Aliphatic-Carbon, A-Aromatic-Carbon
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Alkane-and-EWG2-Nitrite-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon
R4 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
# Nucleophilic-Aliphatic-Substitution-Acidic-Lg-Chlorine-and-Nu-Azide
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Reactions of Azides - Substitution, Reduction, Rearrangements, and More
Condition to enforce:
R1 = A-Aliphatic-Carbon
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carboxyl-and-EWG2-Carboxyl-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
# Nucleophilic-Substitution-Enamine-Lg-Bromine
References:
[0]
Enamine - Wikipedia
Condition to enforce:
R1 = H, A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon
R4 = H, A-Aliphatic-Carbon
R5 = A-Aliphatic-Carbon
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Bromine-and-Nu-Carboxyl-H
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
carboxylic-acid-as-nucleophile
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carboxyl-and-EWG2-Nitrite-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
R4 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
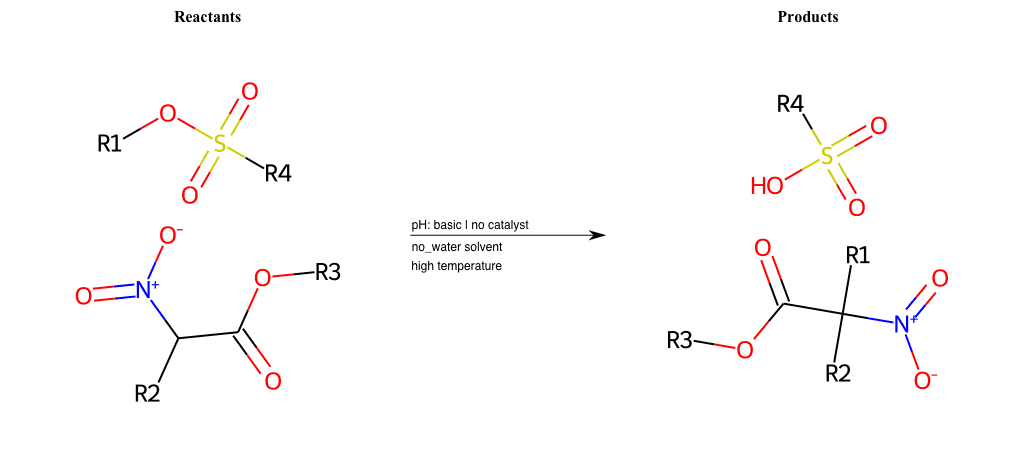
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Bromine-and-Nu-Sulfonamide
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
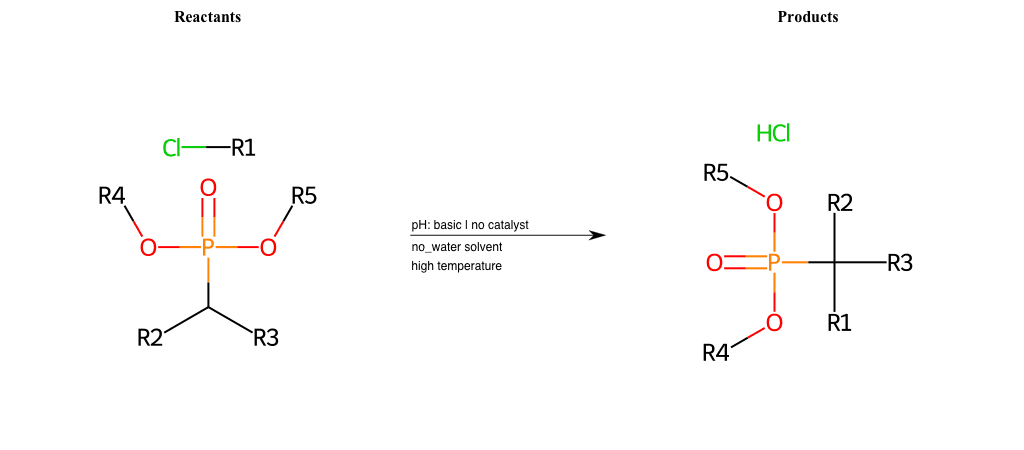
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Alkane-and-EWG2-Phosphonate-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R5 = A-Aliphatic-Carbon, A-Aromatic-Carbon
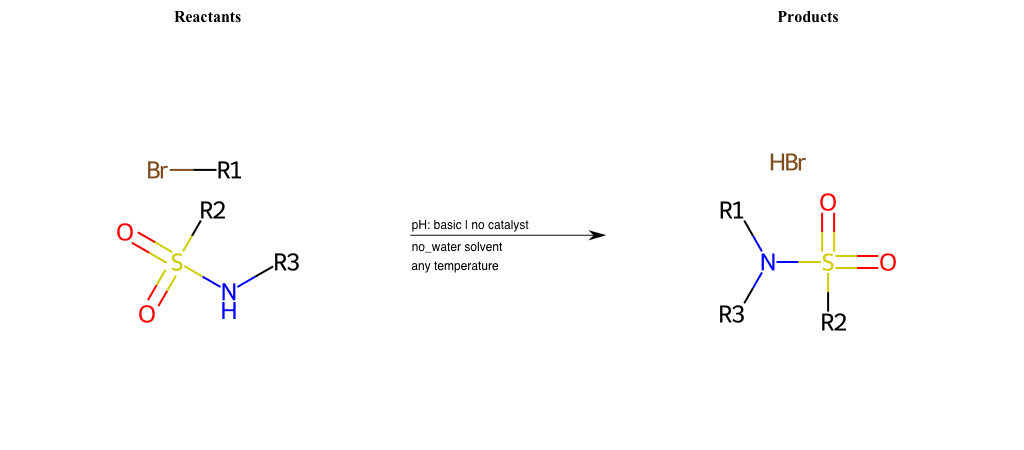
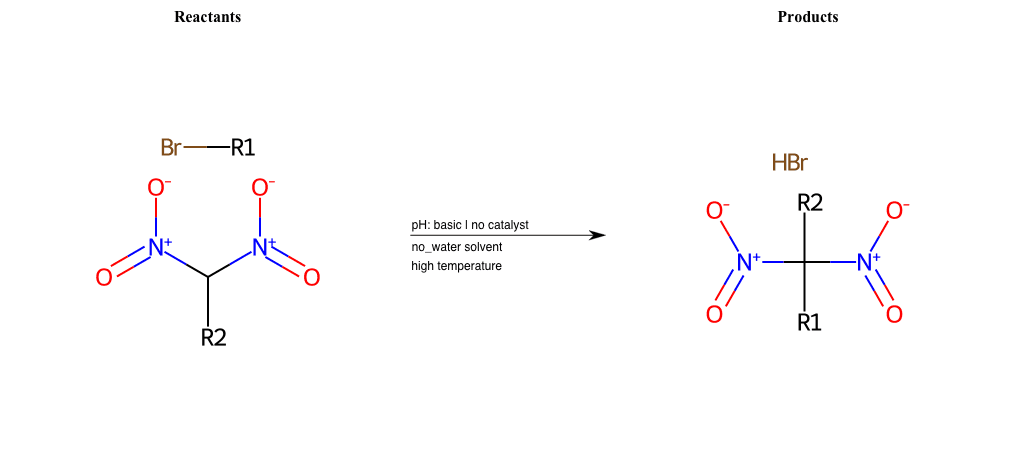
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Nitrite-and-EWG2-Nitrite-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
# Epoxide-Ring-Opening-Nu-Carboxyl-H-basic
References:
[0]
Epoxidation - Chemistry LibreTexts
[1]
Epoxides Ring-Opening Reactions - Chemistry Steps
Condition to enforce:
R1 = L-A, A-Aromatic-Carbon
R2 = L-A, A-Aromatic-Carbon
R3 = L-A, A-Aromatic-Carbon
R4 = L-A, A-Aromatic-Carbon
R5 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
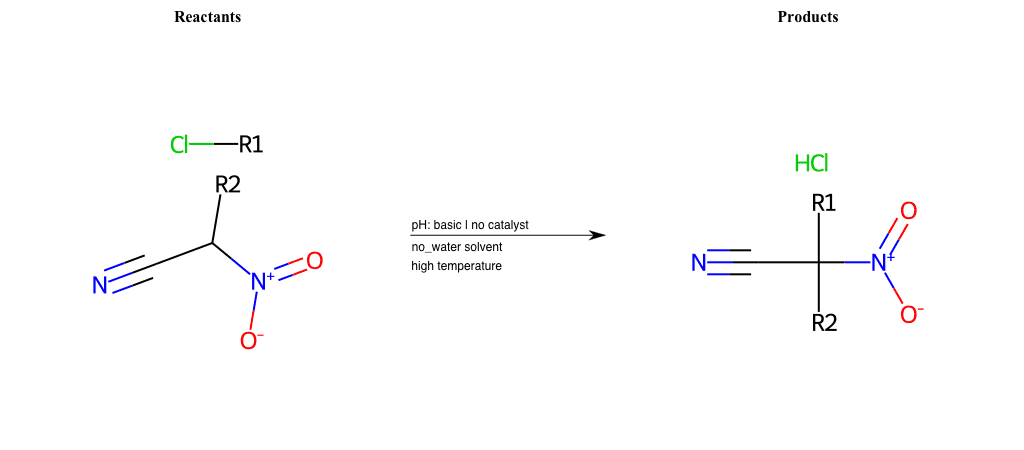
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Nitrile-and-EWG2-Nitrite-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
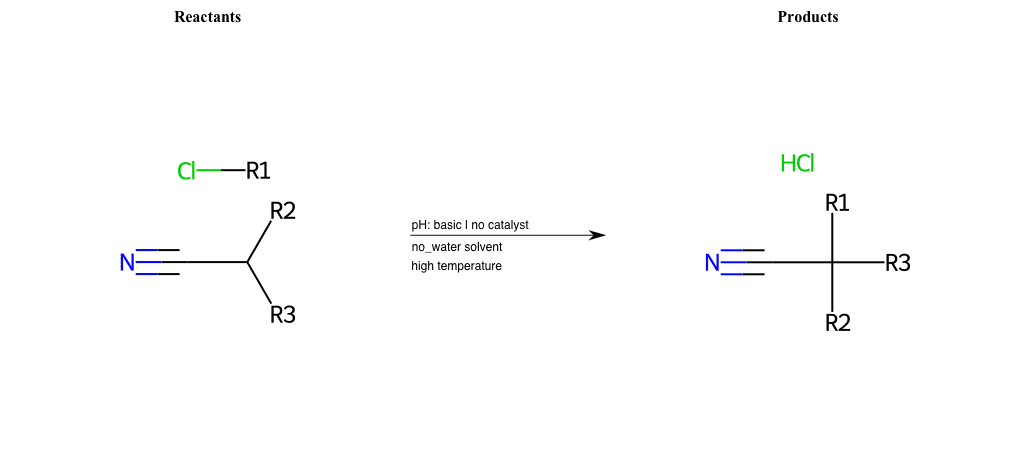
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Alkane-and-EWG2-Nitrile-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon
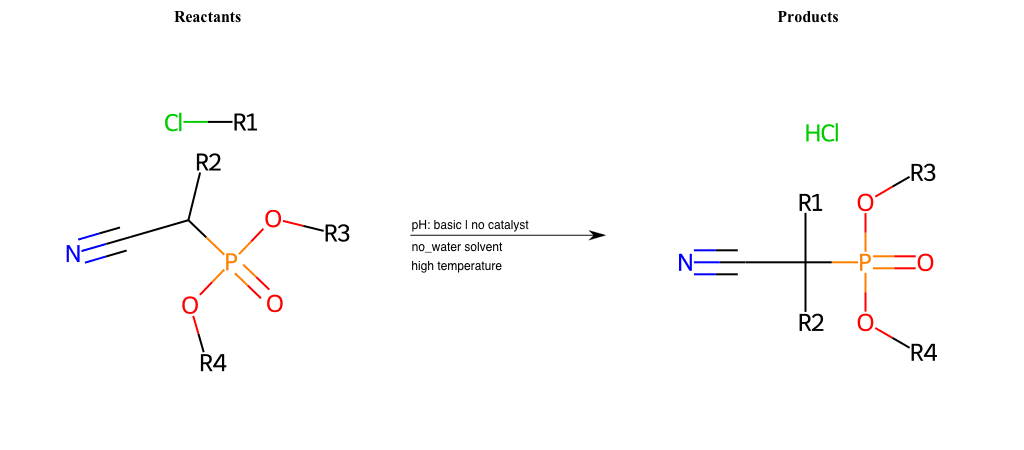
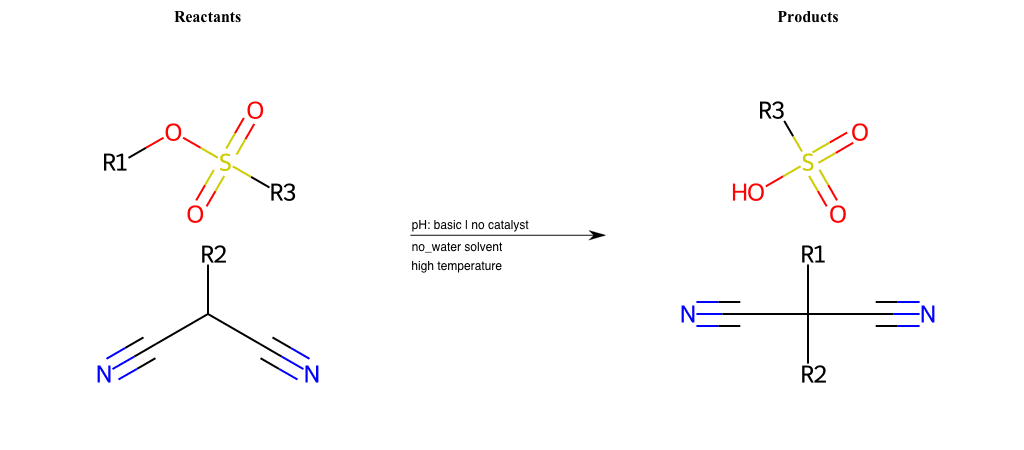
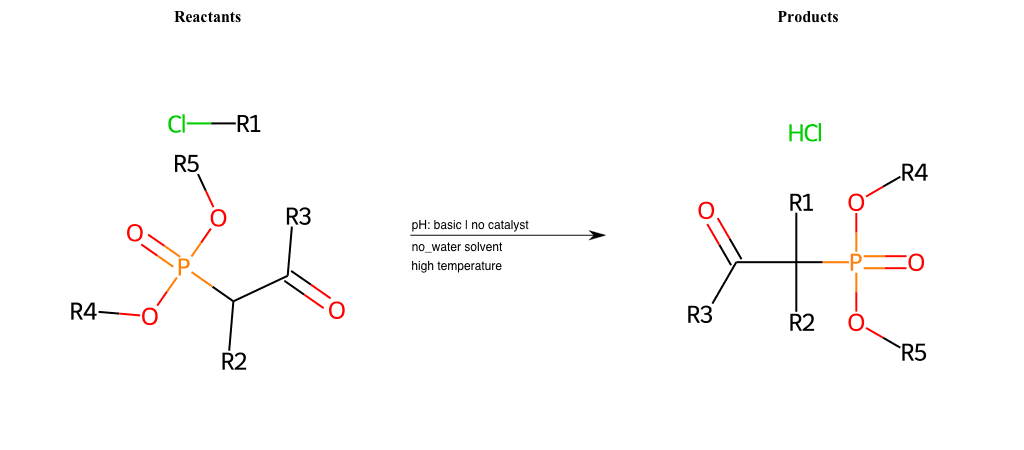
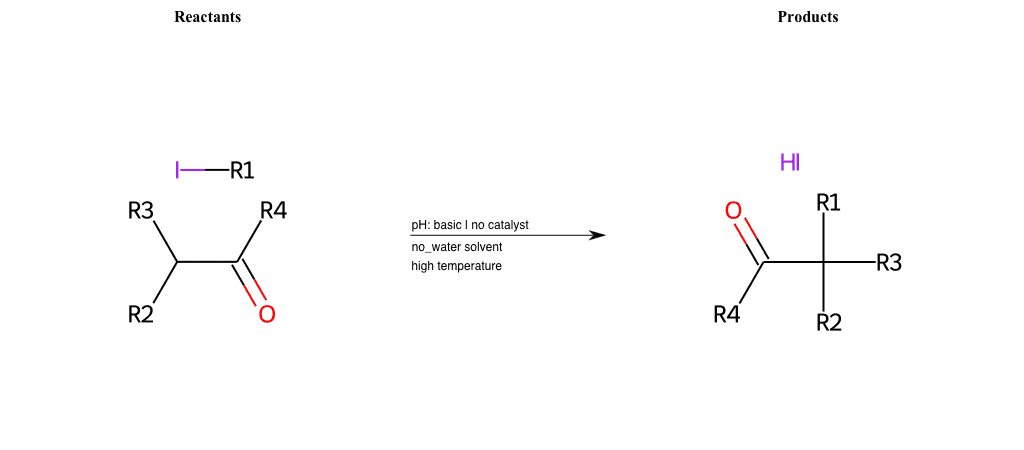
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Nitrile-and-EWG2-Phosphonate-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
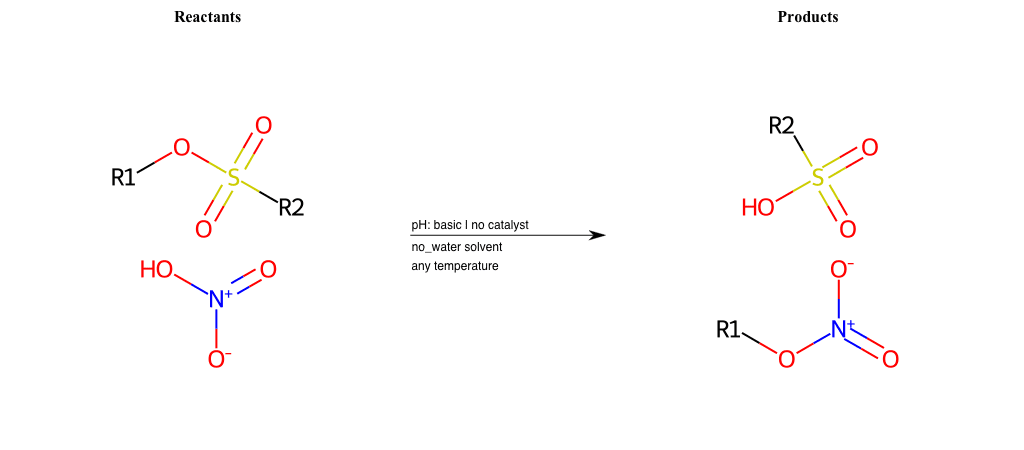
R2 = H, A-Aliphatic-Carbon
R3 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carboxyl-and-EWG2-Carboxyl-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
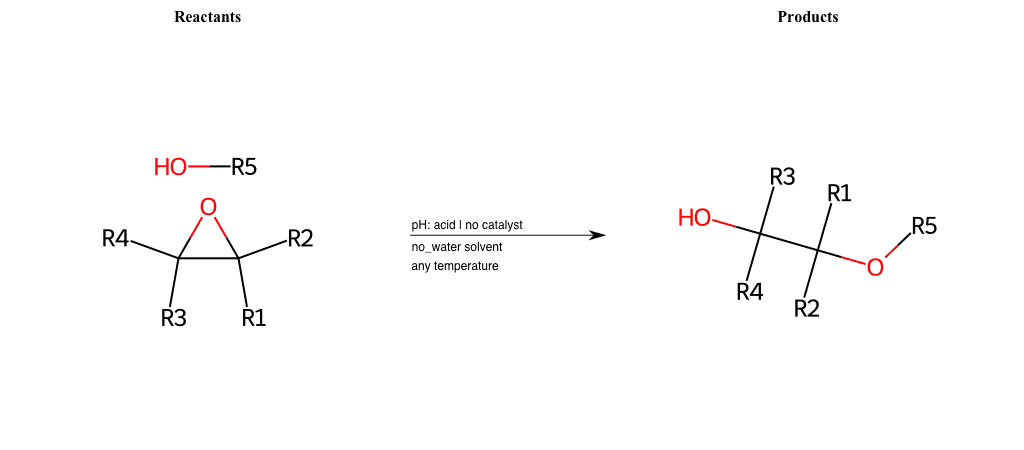
# Epoxide-Ring-Opening-Nu-Alkoxide-acid
References:
[0]
Epoxidation - Chemistry LibreTexts
[1]
Epoxides Ring-Opening Reactions - Chemistry Steps
Condition to enforce:
R1 = L-A, A-Aromatic-Carbon
R2 = L-A, A-Aromatic-Carbon
R3 = L-A, A-Aromatic-Carbon
R4 = L-A, A-Aromatic-Carbon
R5 = A-Aliphatic-Carbon, A-Aromatic-Carbon
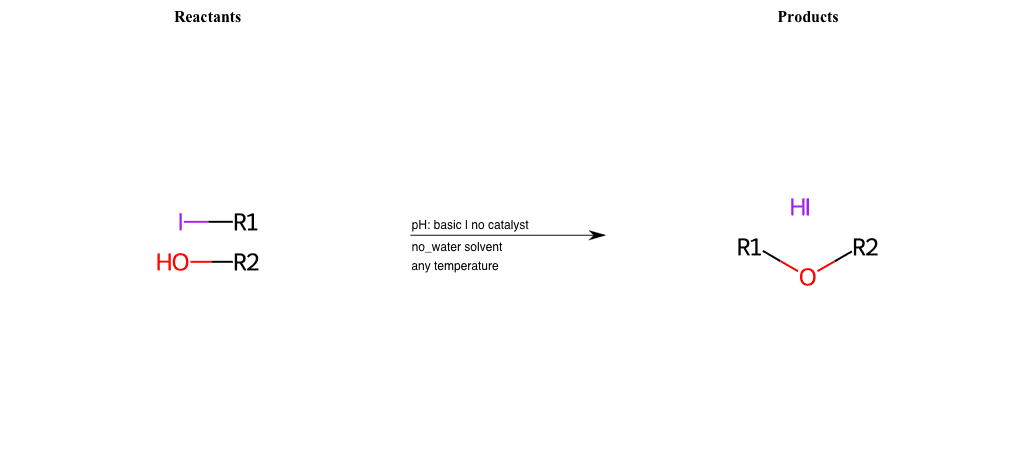
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Iodine-and-Nu-Alkoxide
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Williamson ether synthesis - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = A-Aliphatic-Carbon, A-Aromatic-Carbon
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Alkane-and-EWG2-Nitrite-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Iodine-and-Nu-N-Carbamate
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
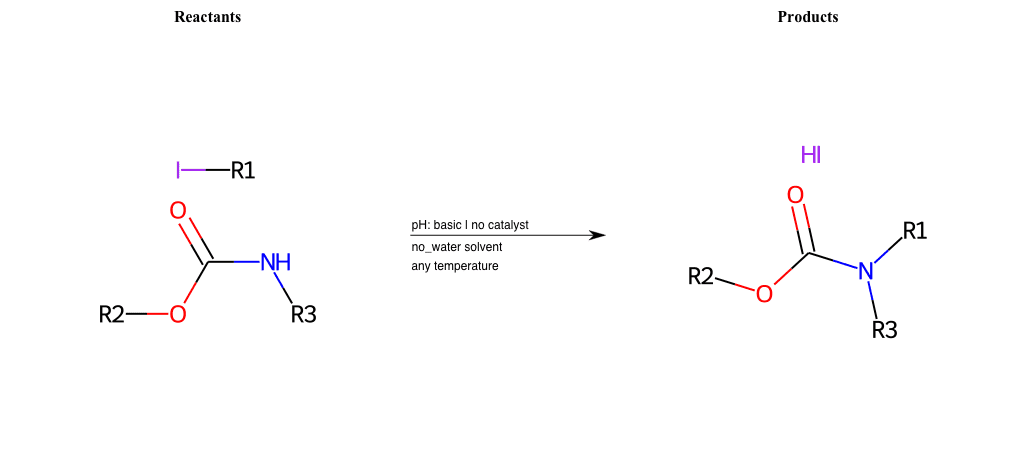
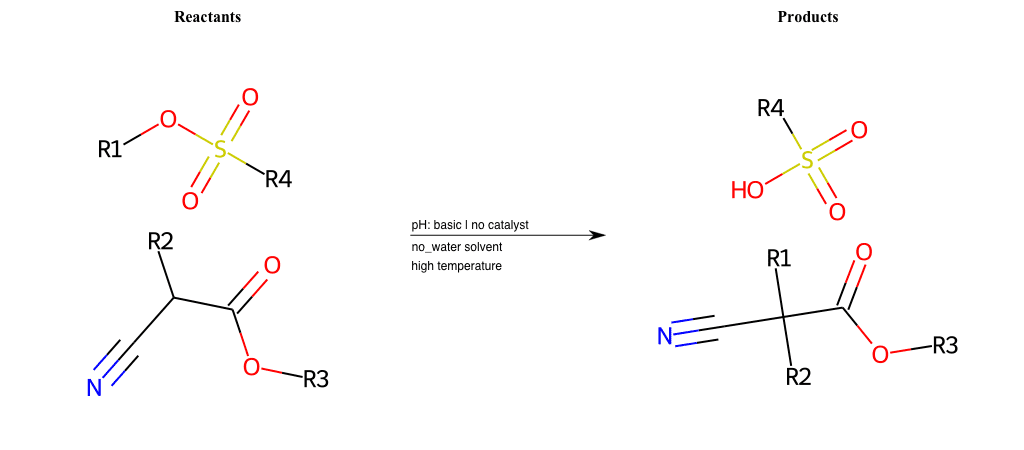
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carboxyl-and-EWG2-Nitrile-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
R4 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
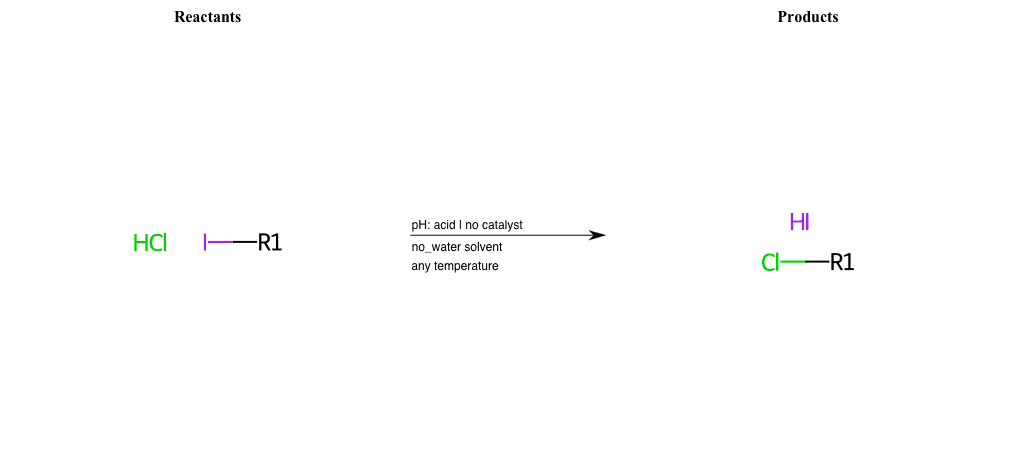
# Nucleophilic-Aliphatic-Substitution-Acidic-Lg-Iodine-and-Nu-Chlorine
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
Condition to enforce:
R1 = A-Aliphatic-Carbon
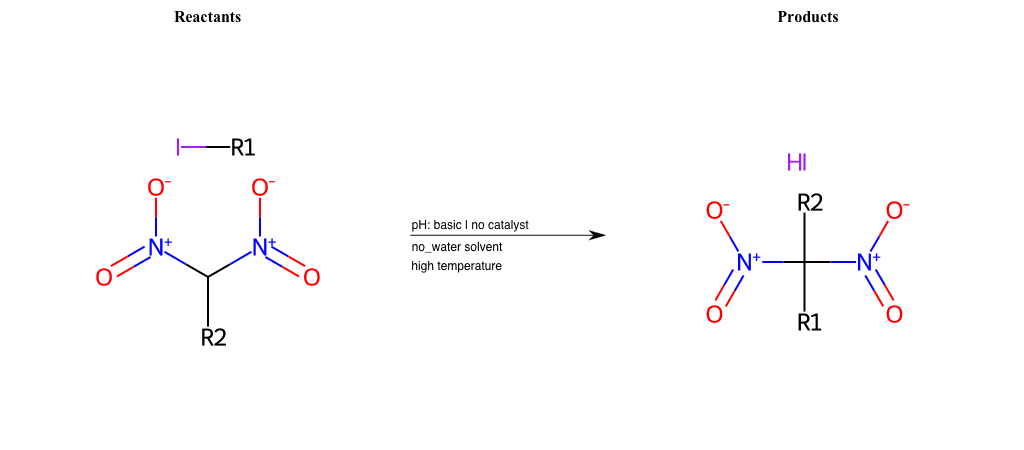
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Nitrite-and-EWG2-Nitrite-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
# Epoxide-Ring-Opening-Nu-Alkoxide-basic
References:
[0]
Epoxidation - Chemistry LibreTexts
[1]
Epoxides Ring-Opening Reactions - Chemistry Steps
Condition to enforce:
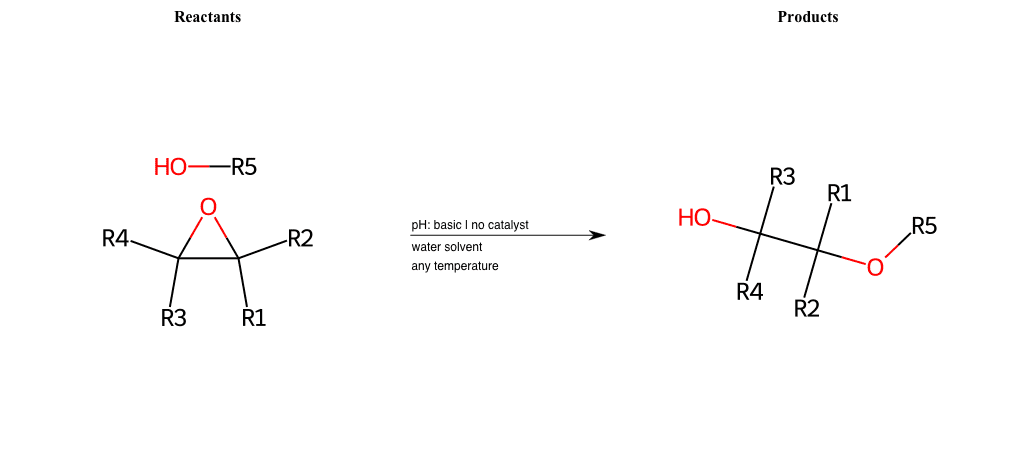
R1 = L-A, A-Aromatic-Carbon
R2 = L-A, A-Aromatic-Carbon
R3 = L-A, A-Aromatic-Carbon
R4 = L-A, A-Aromatic-Carbon
R5 = A-Aliphatic-Carbon, A-Aromatic-Carbon
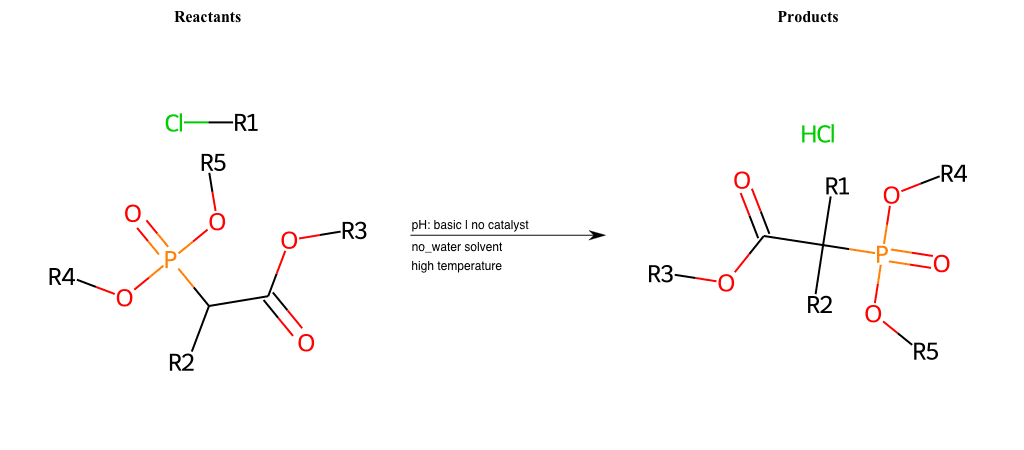
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carboxyl-and-EWG2-Phosphonate-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R5 = A-Aliphatic-Carbon, A-Aromatic-Carbon
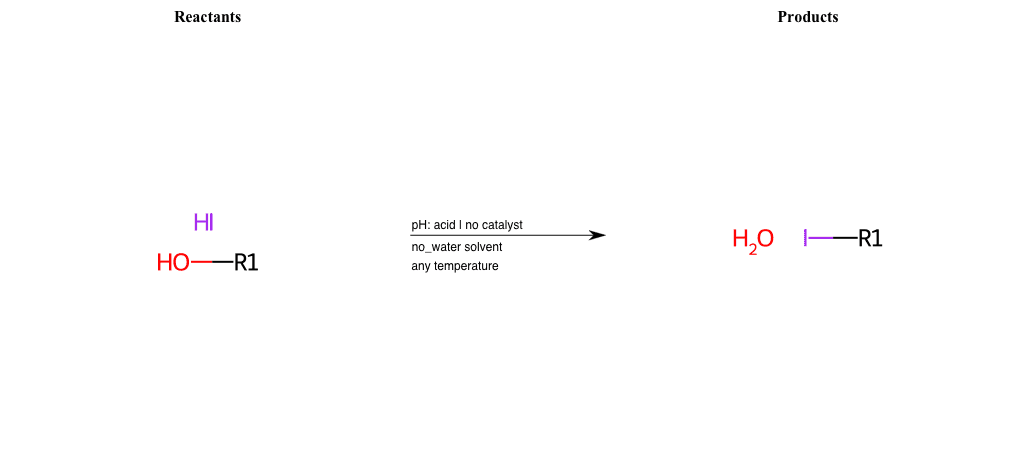
# Nucleophilic-Aliphatic-Substitution-Acidic-Lg-Hydroxyl-and-Nu-Iodine
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Making Alkyl Halides From Alcohols – Master Organic Chemistry
[4]
Ch15 : Alcohols with hydrogen halides to alkyl halides
Condition to enforce:
R1 = A-Aliphatic-Carbon
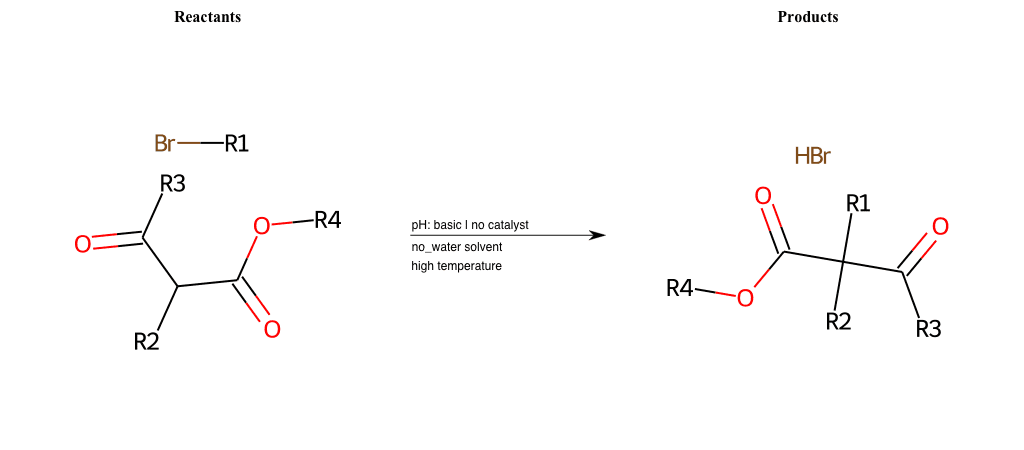
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carbonyl-and-EWG2-Carboxyl-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Alkane-and-EWG2-Carbonyl-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon
R4 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
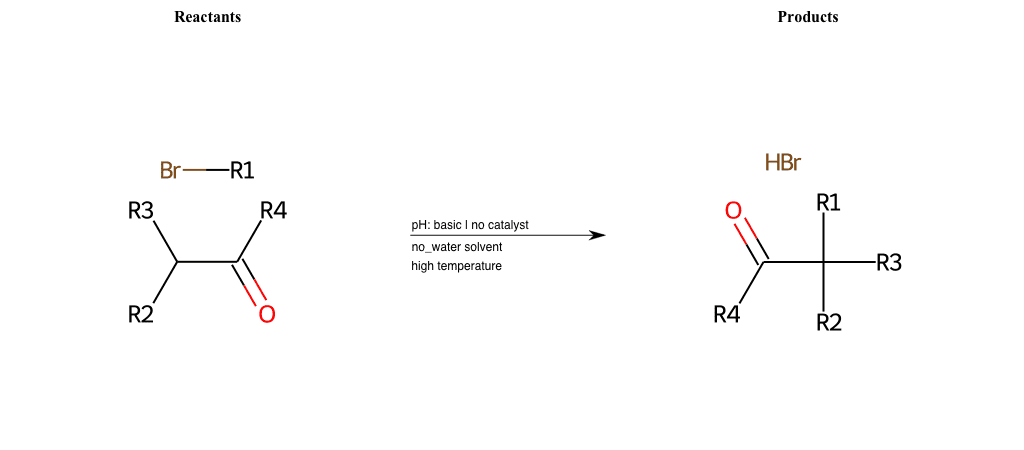
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Alkane-and-EWG2-Phosphonate-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R5 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
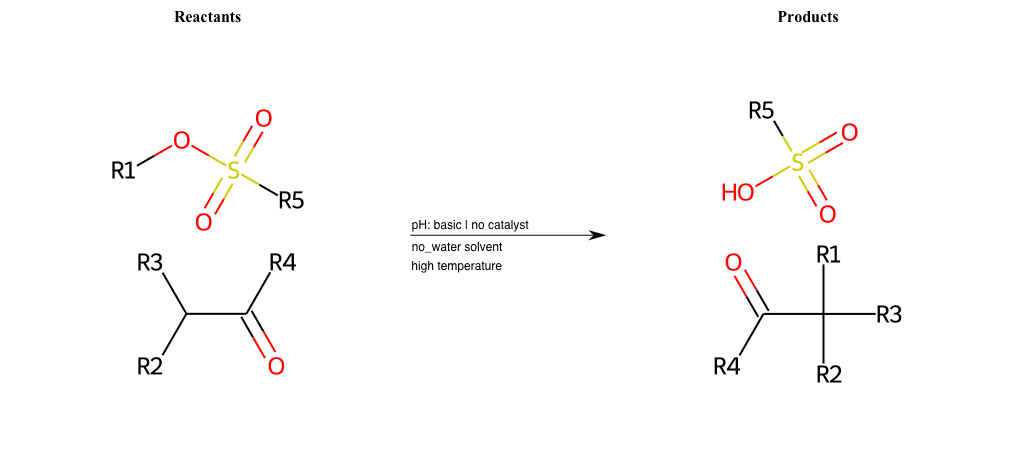
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Alkane-and-EWG2-Carbonyl-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon
R4 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R5 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
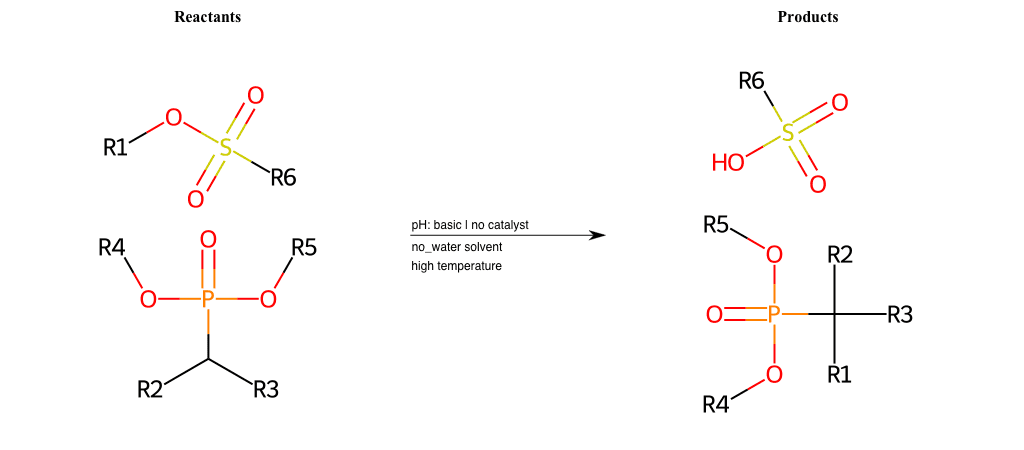
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Sulfonate-and-Nu-Sulfonamide
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R4 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
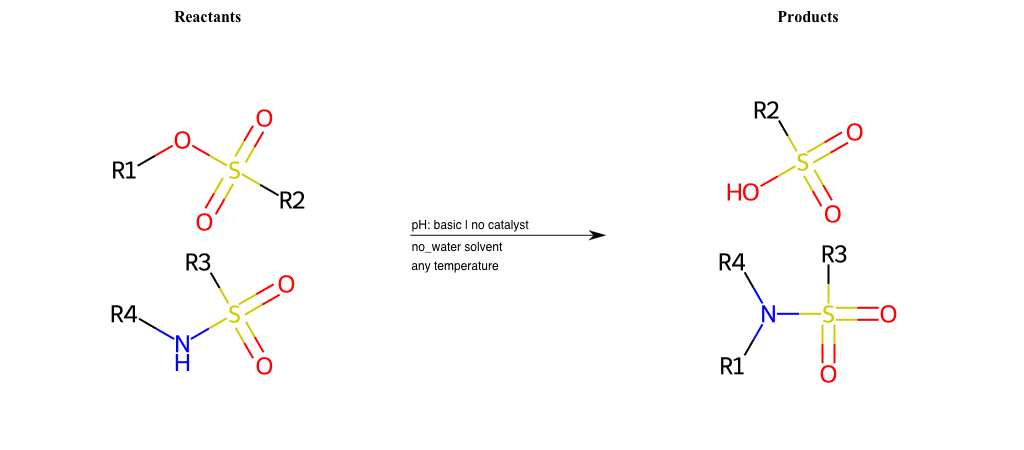
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Chlorine-and-Nu-Thiolate
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = A-Aliphatic-Carbon, A-Aromatic-Carbon
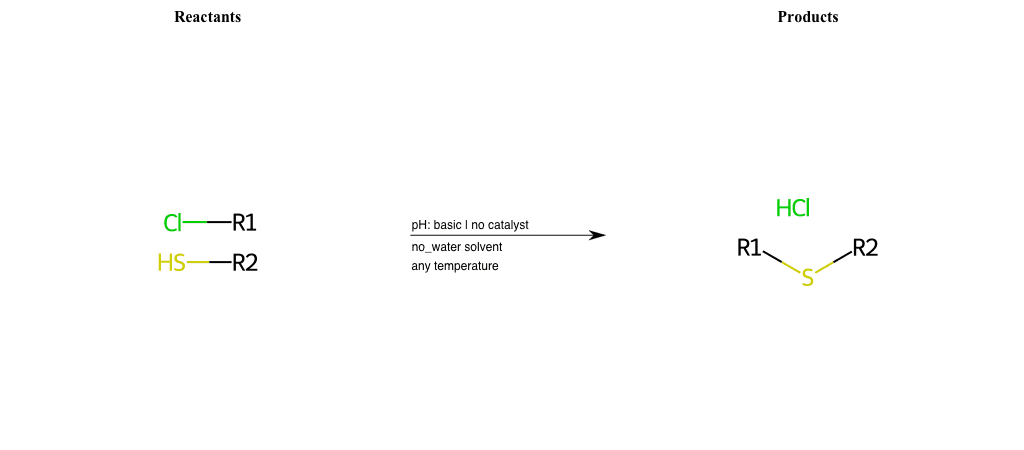
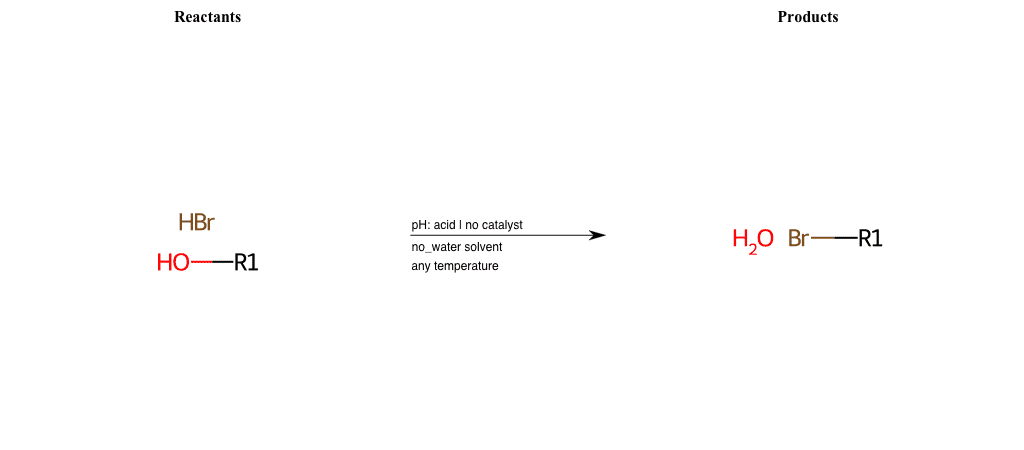
# Nucleophilic-Aliphatic-Substitution-Acidic-Lg-Hydroxyl-and-Nu-Bromine
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Making Alkyl Halides From Alcohols – Master Organic Chemistry
[4]
Ch15 : Alcohols with hydrogen halides to alkyl halides
Condition to enforce:
R1 = A-Aliphatic-Carbon
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Bromine-and-Nu-Thiolate
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = A-Aliphatic-Carbon, A-Aromatic-Carbon
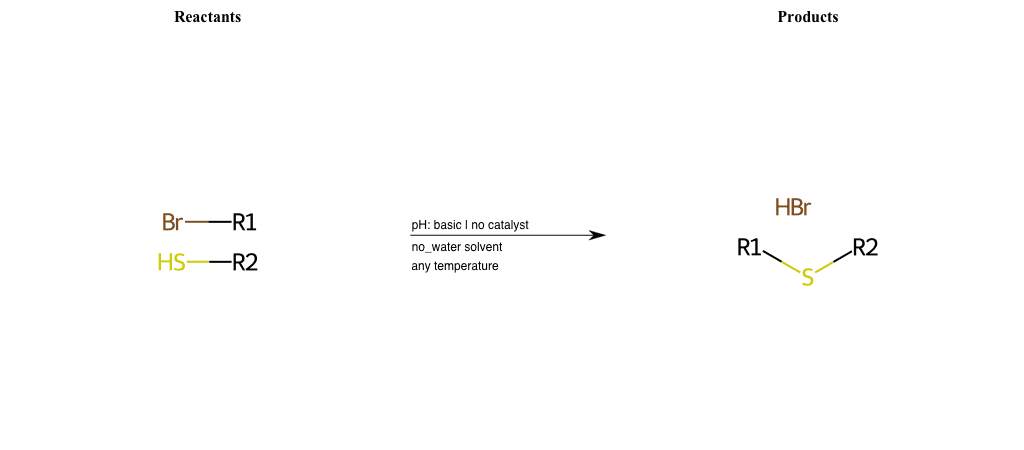
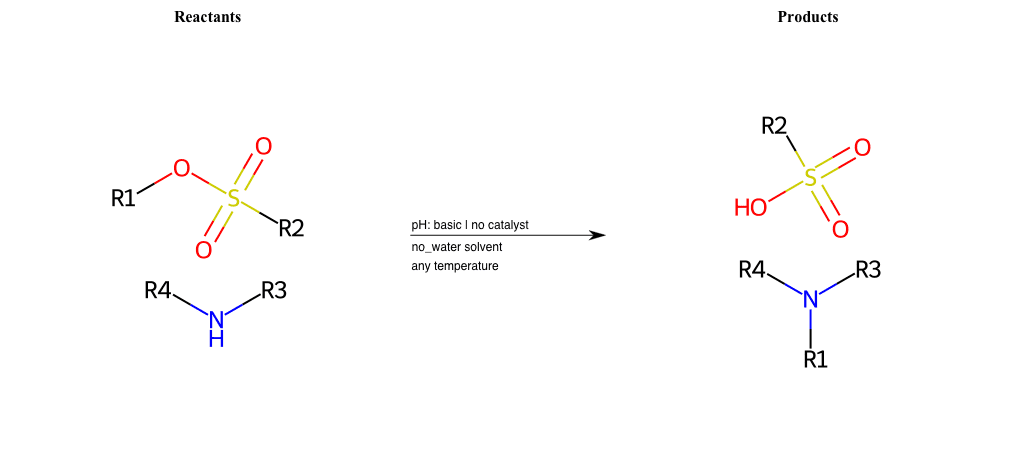
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Sulfonate-and-Nu-Amino
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Alkylation of Amines (Sucks!) – Master Organic Chemistry
[4]
9.4. Reaction of RX with NH3 and amines | Organic Chemistry 1: An open textbook
[5]
Amines as Nucleophiles - Chemistry LibreTexts
[6]
Gabriel Phthalimide Synthesis Mechanism - Explanation and Examples
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
R4 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carbonyl-and-EWG2-Nitrite-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R4 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
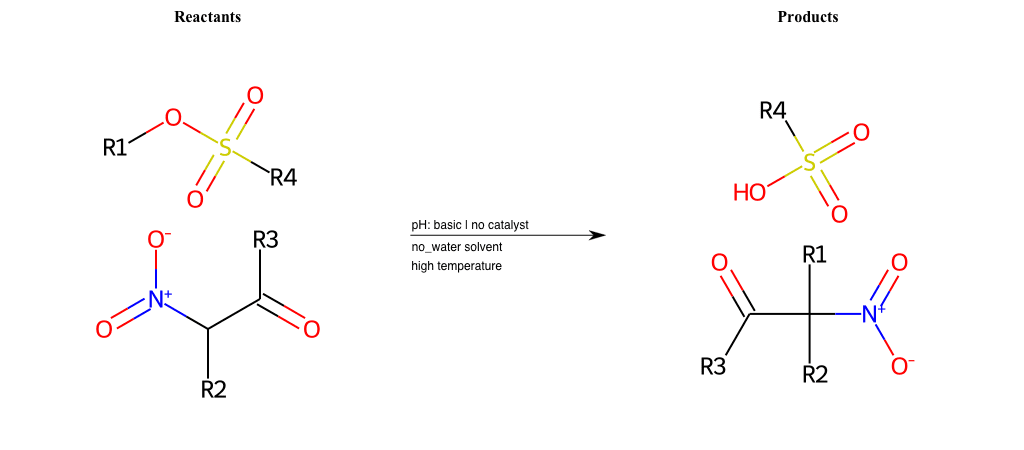
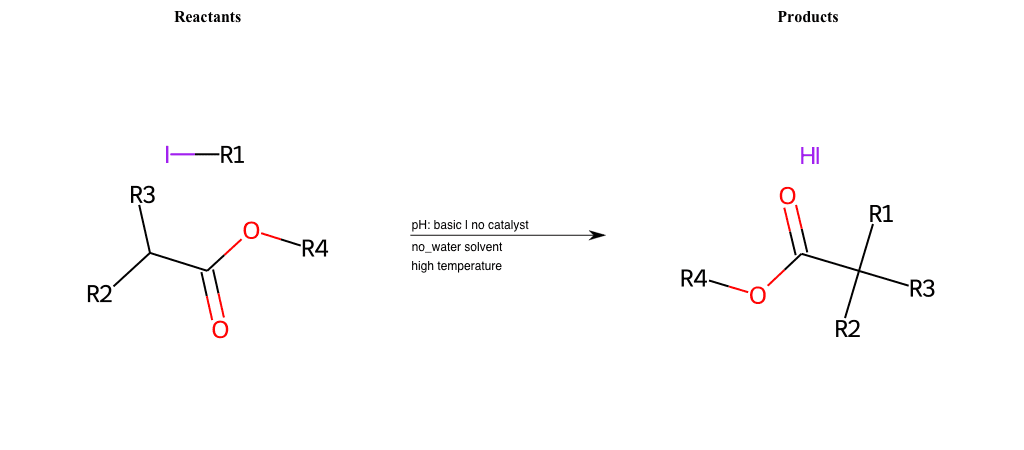
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Alkane-and-EWG2-Carboxyl-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
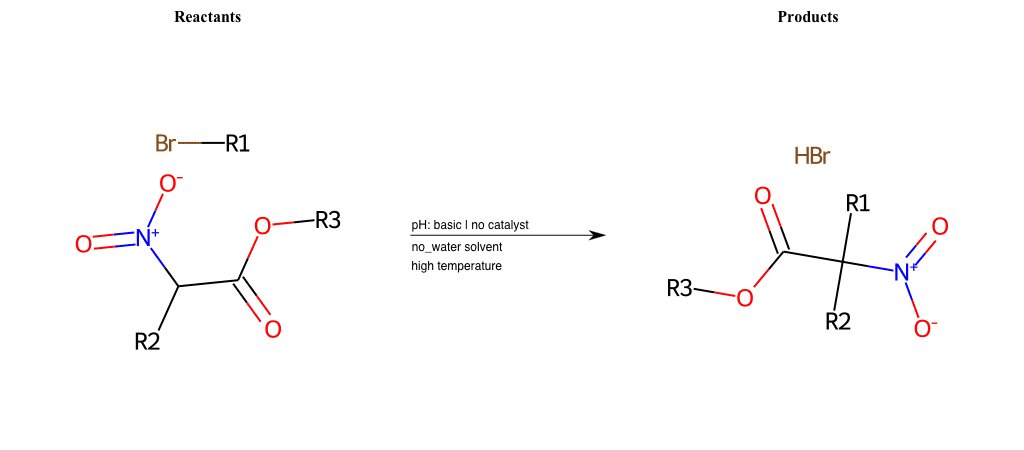
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carboxyl-and-EWG2-Nitrite-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Chlorine-and-Nu-Nitrile
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Kolbe Nitrile Synthesis
[4]
Pelouze Synthesis
Condition to enforce:
R1 = A-Aliphatic-Carbon
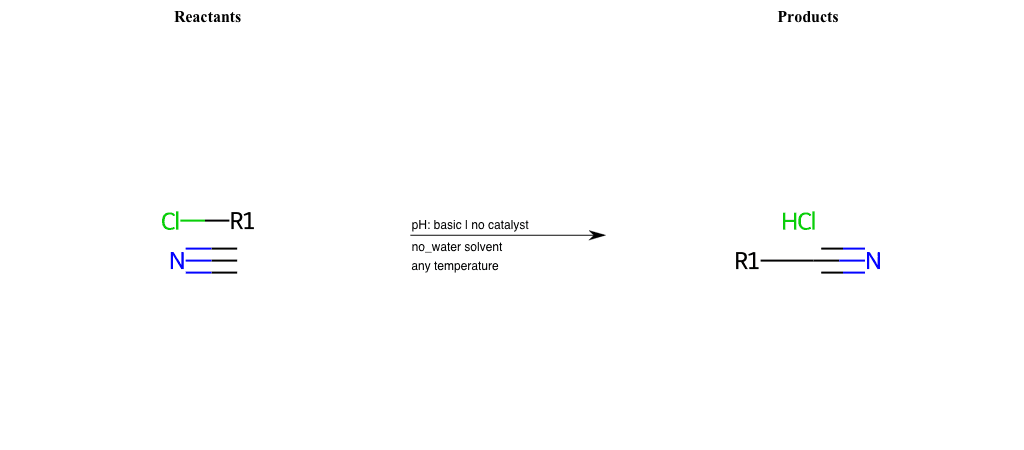
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Nitrite-and-EWG2-Phosphonate-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon
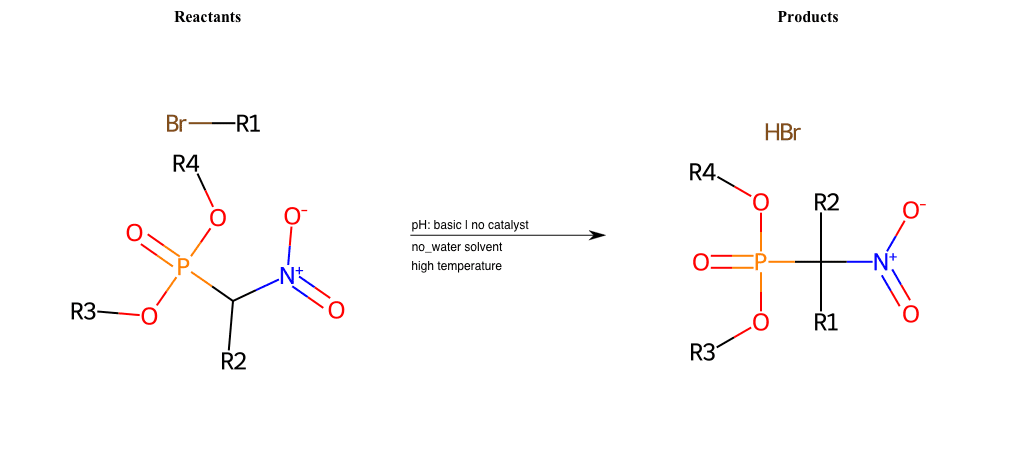
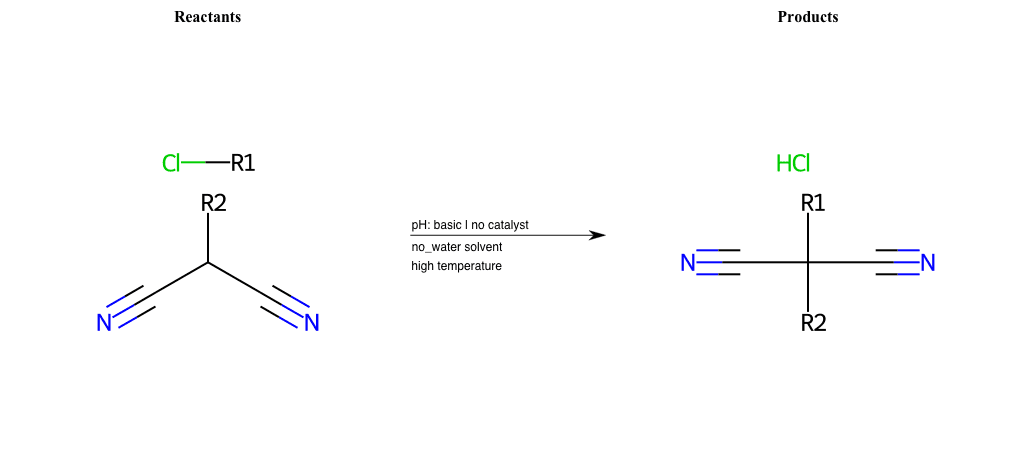
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Nitrile-and-EWG2-Nitrile-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
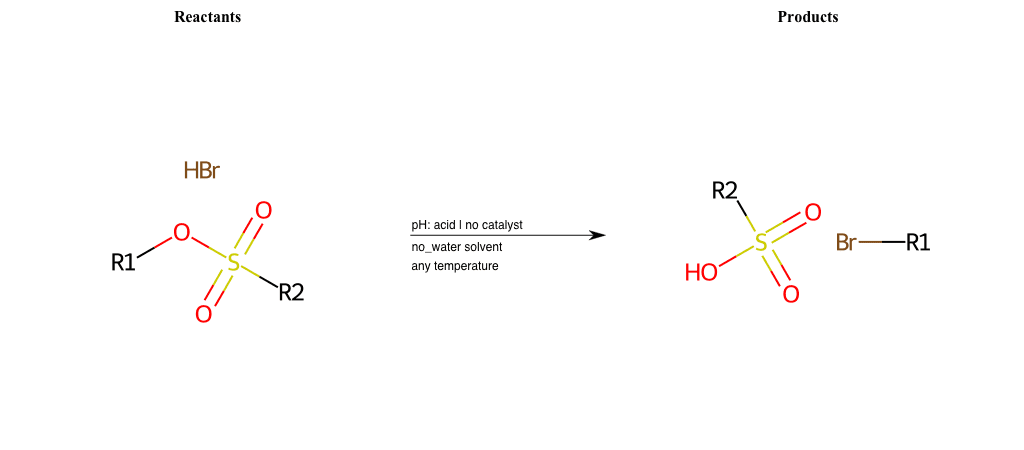
# Nucleophilic-Aliphatic-Substitution-Acidic-Lg-Sulfonate-and-Nu-Bromine
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
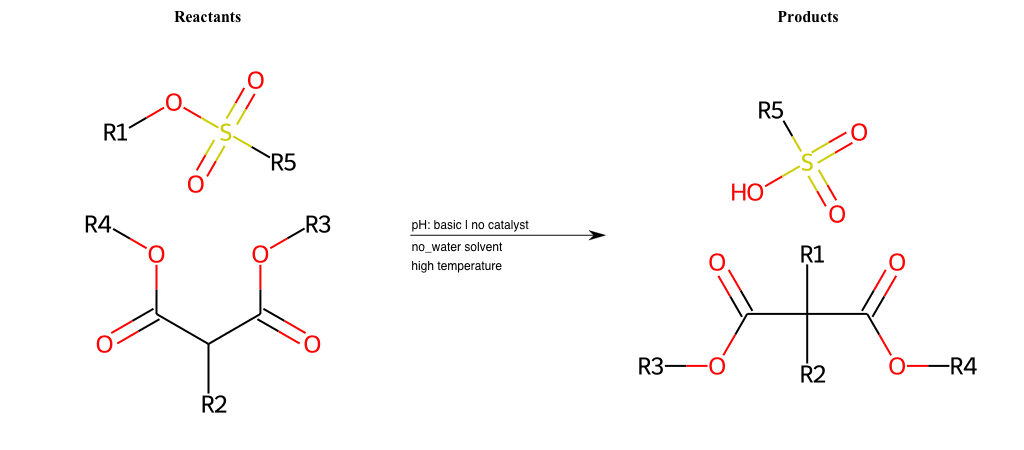
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carboxyl-and-EWG2-Carboxyl-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
R5 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Sulfonate-and-Nu-Nitrate
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Nitrile-and-EWG2-Nitrile-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carbonyl-and-EWG2-Phosphonate-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R5 = A-Aliphatic-Carbon, A-Aromatic-Carbon
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Alkane-and-EWG2-Carbonyl-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon
R4 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Alkane-and-EWG2-Nitrile-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon
R4 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
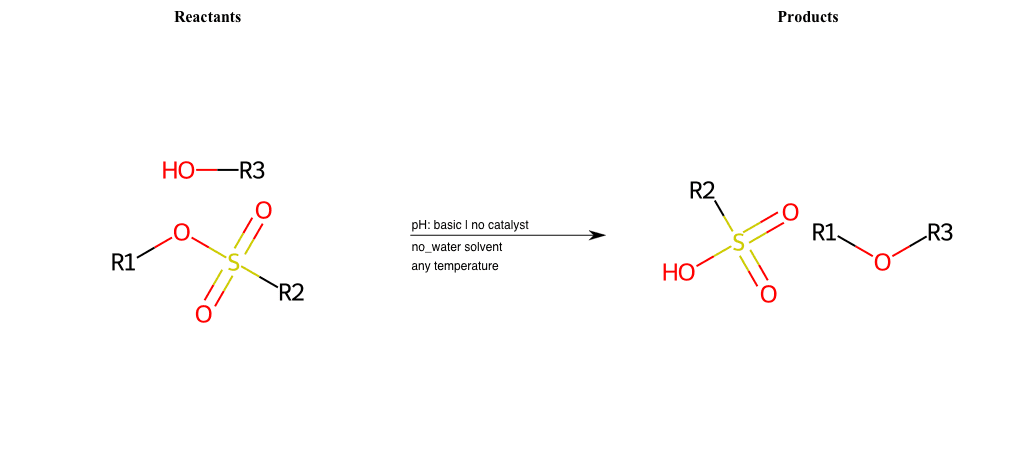
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Sulfonate-and-Nu-Alkoxide
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Williamson ether synthesis - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R3 = A-Aliphatic-Carbon, A-Aromatic-Carbon
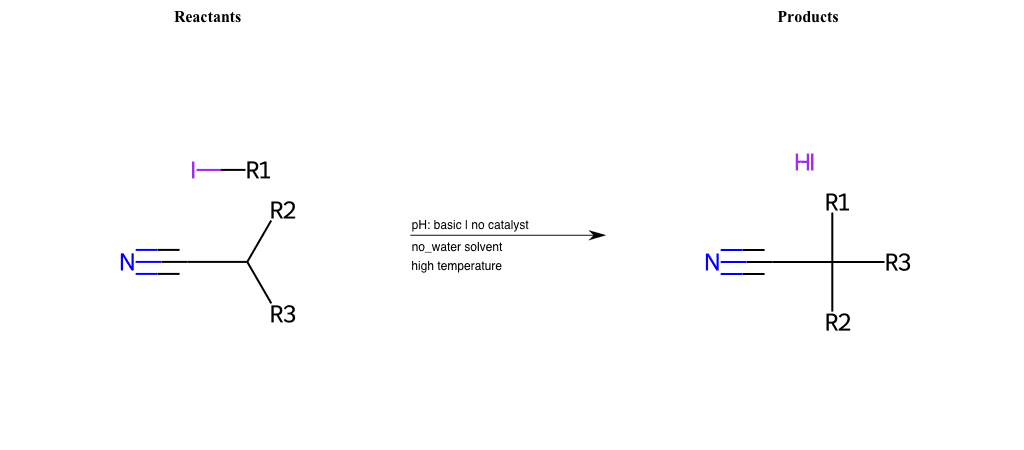
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Iodine-and-Nu-Nitrate
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
Condition to enforce:
R1 = A-Aliphatic-Carbon
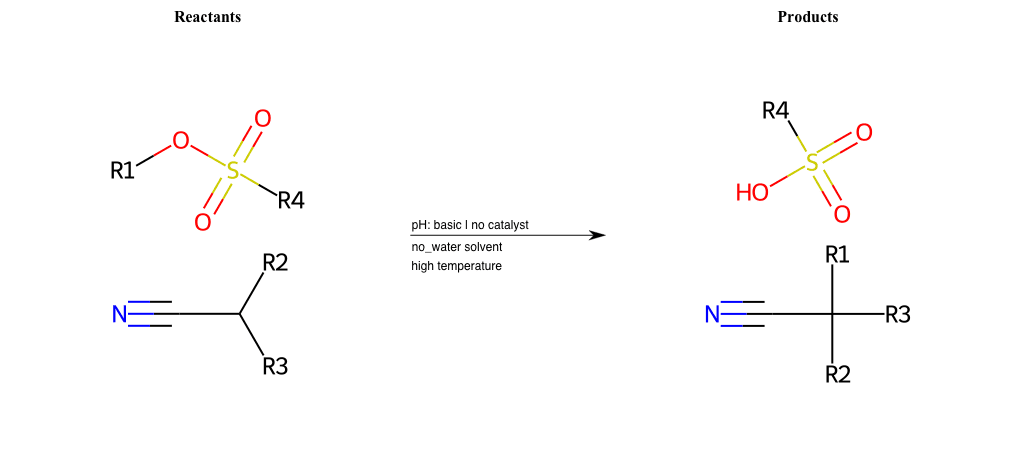
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Alkane-and-EWG2-Nitrile-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon
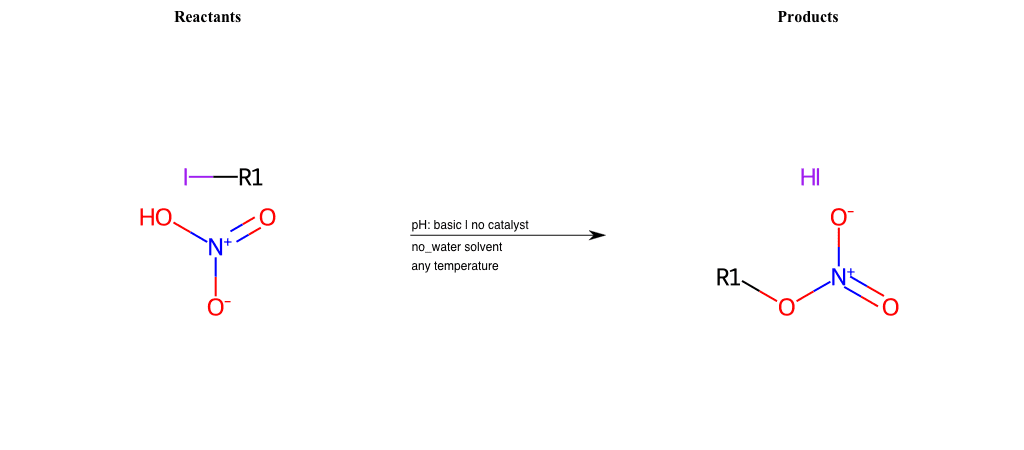
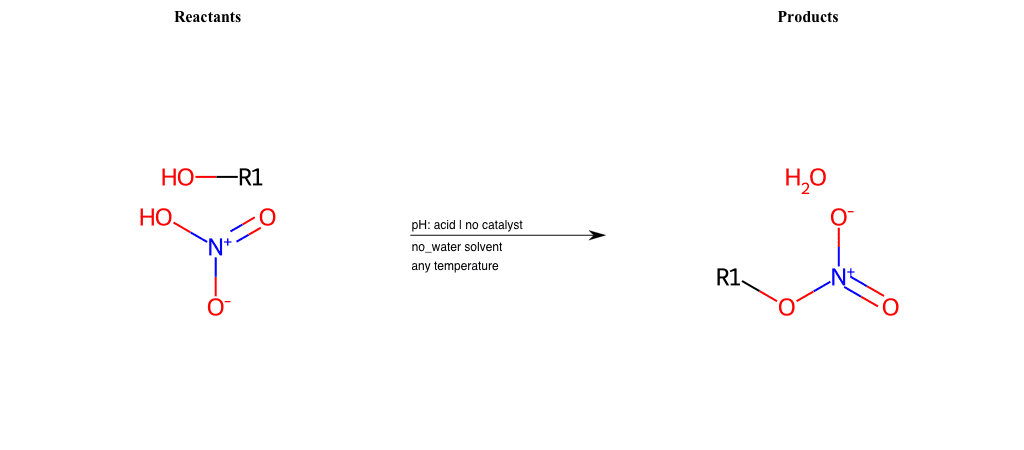
# Nucleophilic-Aliphatic-Substitution-Acidic-Lg-Hydroxyl-and-Nu-Nitrate
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Nitrate ester - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
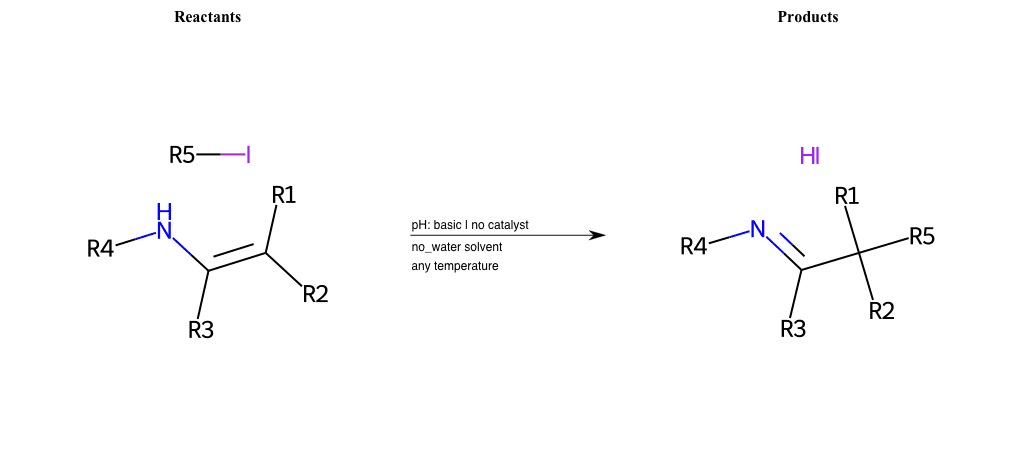
# Nucleophilic-Substitution-Enamine-Lg-Iodine
References:
[0]
Enamine - Wikipedia
Condition to enforce:
R1 = H, A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon
R4 = H, A-Aliphatic-Carbon
R5 = A-Aliphatic-Carbon
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Nitrile-and-EWG2-Nitrile-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
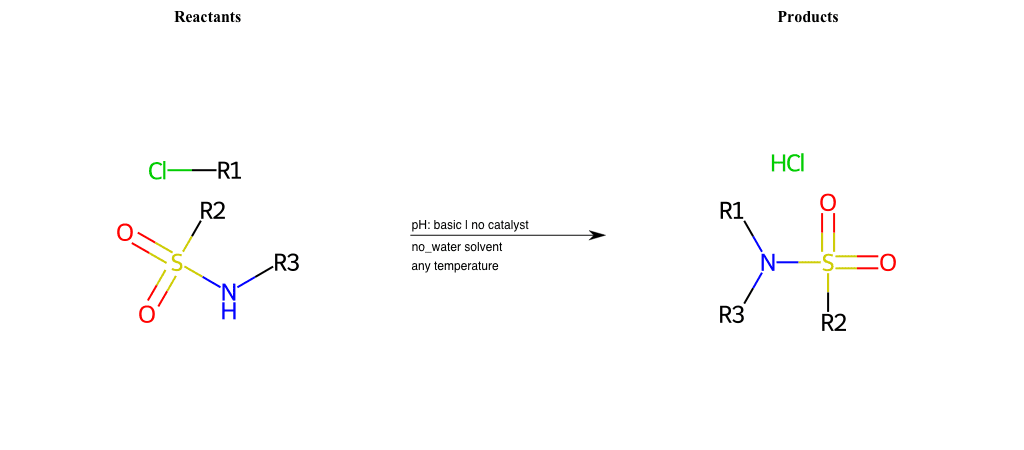
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Chlorine-and-Nu-Sulfonamide
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
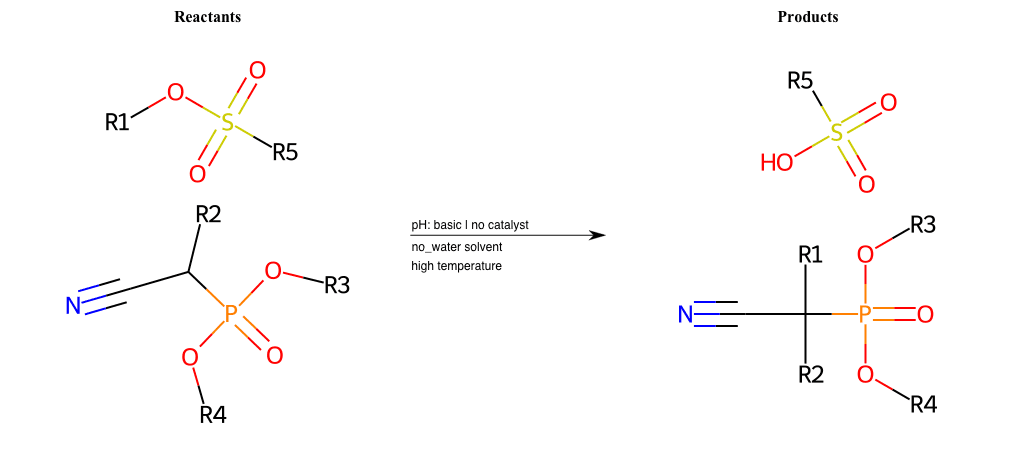
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Nitrile-and-EWG2-Phosphonate-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R5 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
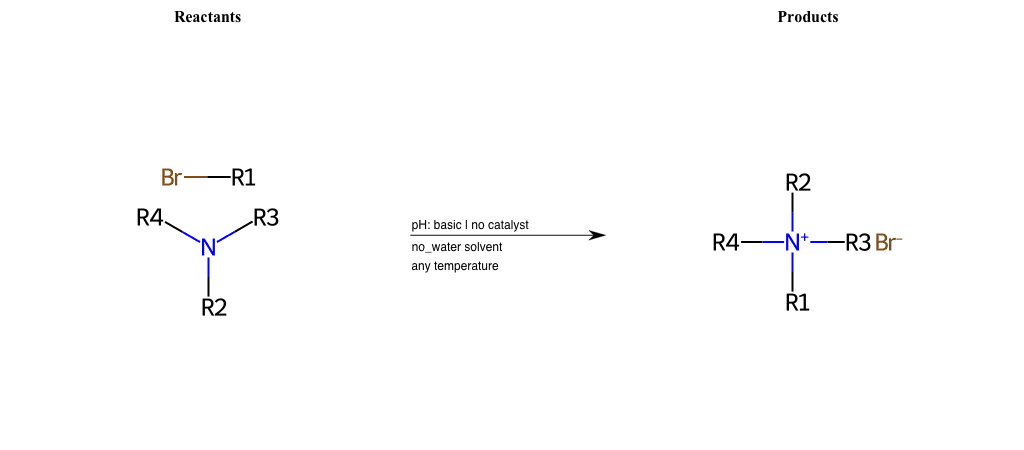
# Nucleophilic-Aliphatic-Substitution-Tertiary-Amine-Lg-Bromine
References:
[0]
Alkylation of Amines (Sucks!) – Master Organic Chemistry
[1]
9.4. Reaction of RX with NH3 and amines | Organic Chemistry 1: An open textbook
[2]
Amines as Nucleophiles - Chemistry LibreTexts
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R3 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon
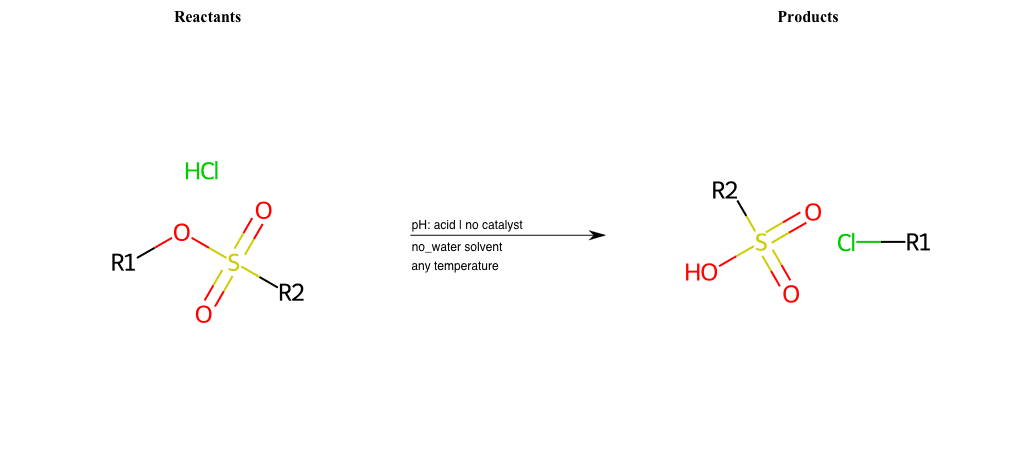
# Nucleophilic-Aliphatic-Substitution-Acidic-Lg-Sulfonate-and-Nu-Chlorine
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
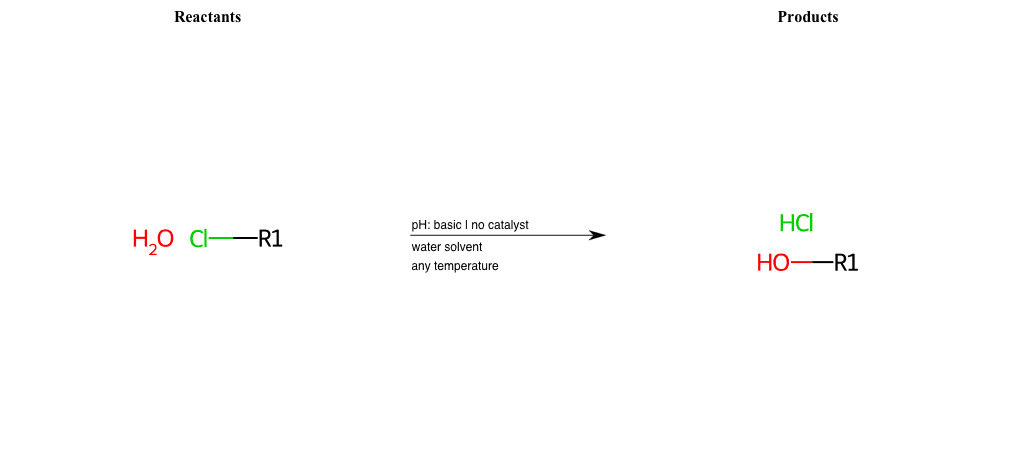
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Chlorine-and-Nu-Hydroxyl
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
Condition to enforce:
R1 = A-Aliphatic-Carbon
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carbonyl-and-EWG2-Nitrile-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R4 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
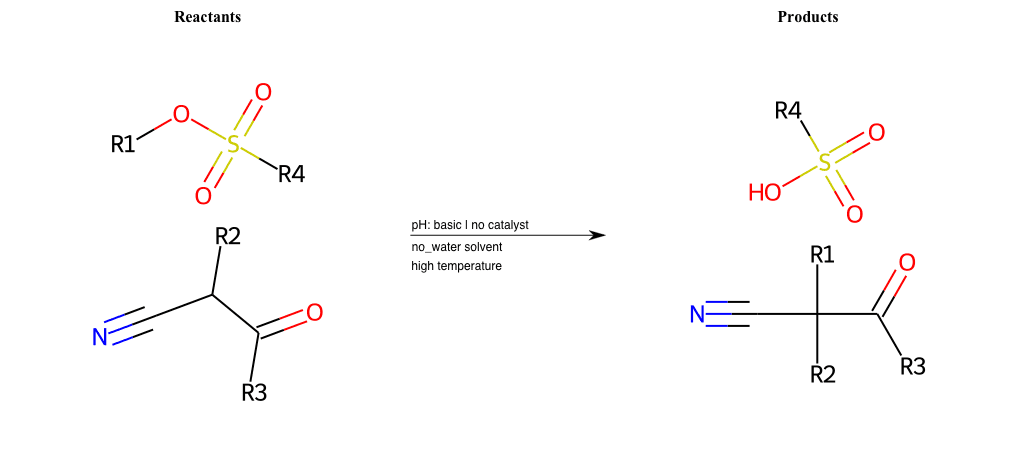
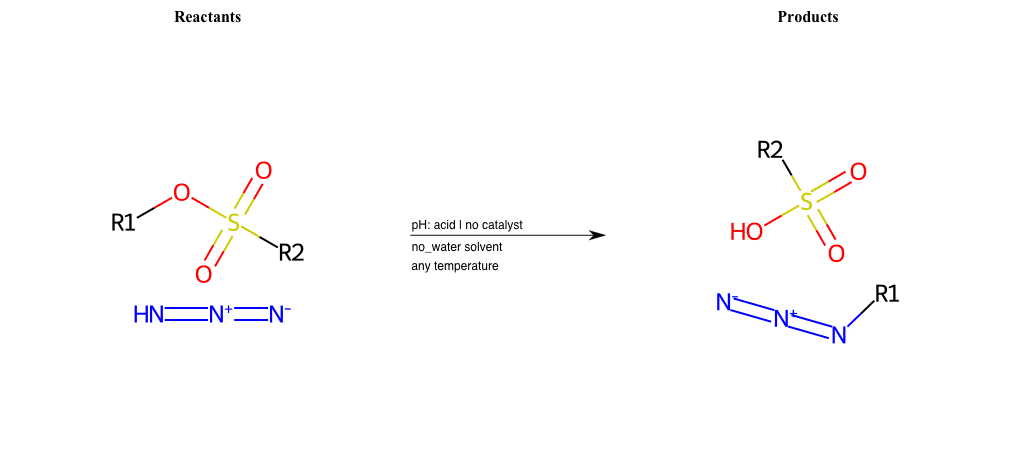
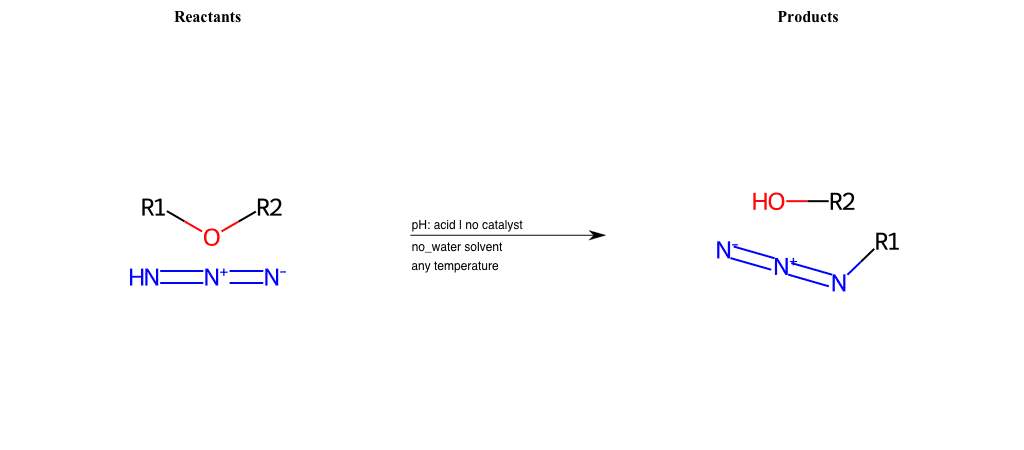
# Nucleophilic-Aliphatic-Substitution-Acidic-Lg-Alkoxide-and-Nu-Azide
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Reactions of Azides - Substitution, Reduction, Rearrangements, and More
[4]
Acidic cleavage of ethers (SN2) – Master Organic Chemistry
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = A-Aliphatic-Carbon
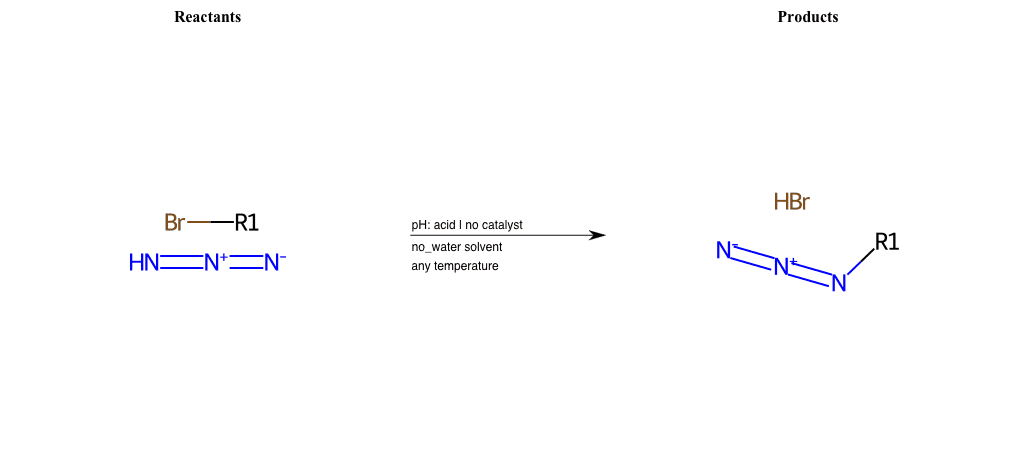
# Nucleophilic-Aliphatic-Substitution-Acidic-Lg-Bromine-and-Nu-Azide
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Reactions of Azides - Substitution, Reduction, Rearrangements, and More
Condition to enforce:
R1 = A-Aliphatic-Carbon
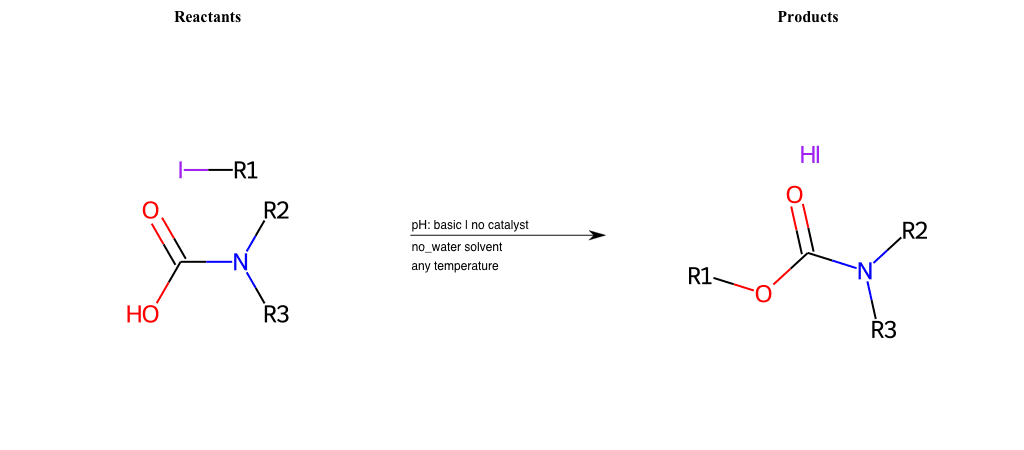
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Iodine-and-Nu-O-Carbamate
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Nitrile-and-EWG2-Nitrite-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
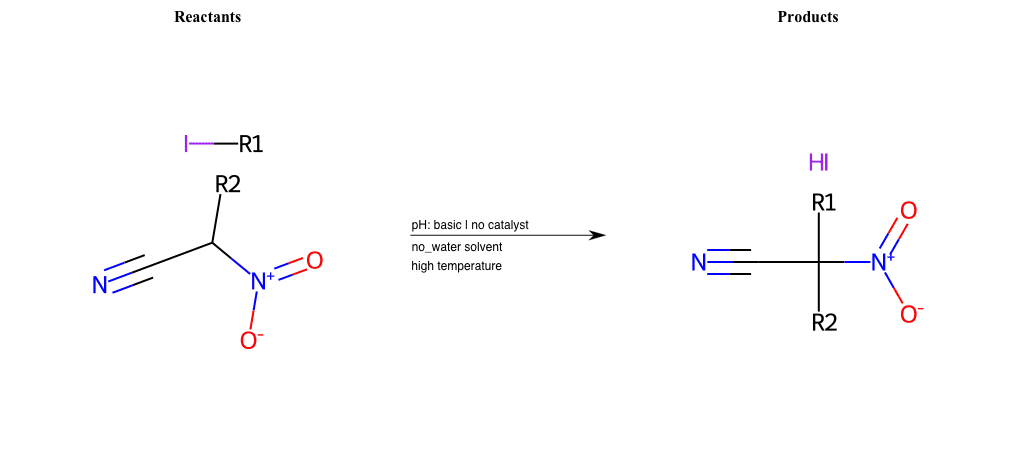
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Bromine-and-Nu-Nitrate
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
Condition to enforce:
R1 = A-Aliphatic-Carbon
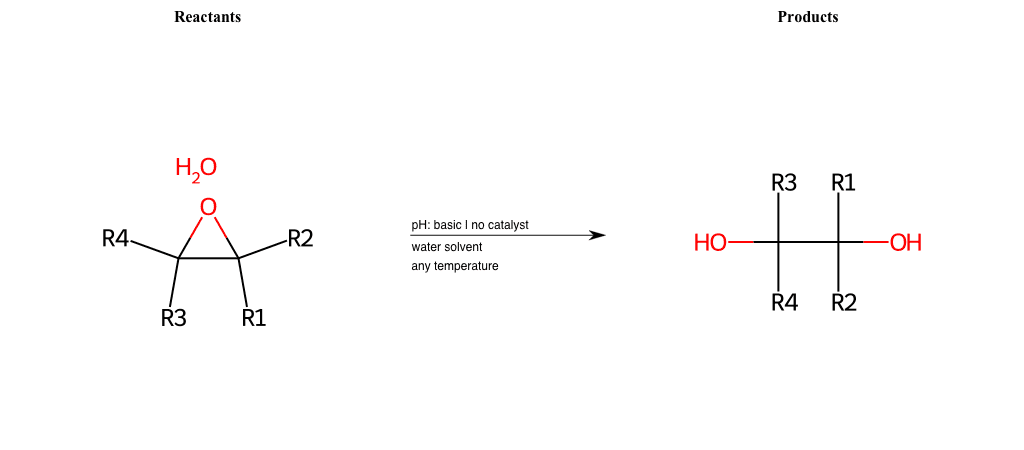
# Epoxide-Ring-Opening-Nu-Hydroxyl-basic
References:
[0]
Epoxidation - Chemistry LibreTexts
[1]
Epoxides Ring-Opening Reactions - Chemistry Steps
Condition to enforce:
R1 = L-A, A-Aromatic-Carbon
R2 = L-A, A-Aromatic-Carbon
R3 = L-A, A-Aromatic-Carbon
R4 = L-A, A-Aromatic-Carbon
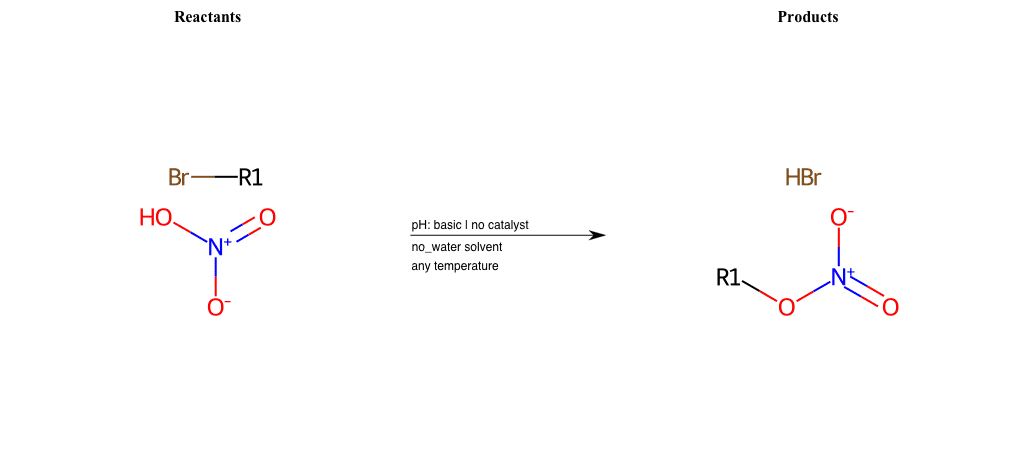
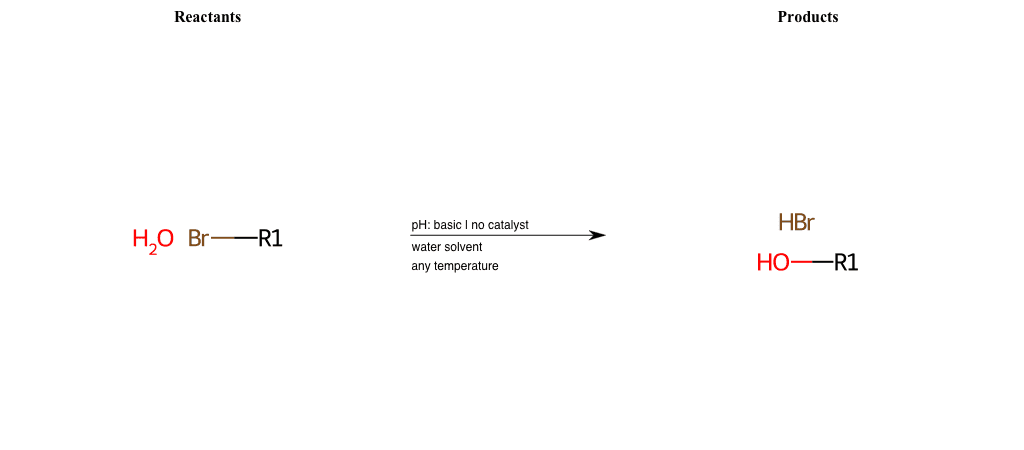
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Bromine-and-Nu-Hydroxyl
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
Condition to enforce:
R1 = A-Aliphatic-Carbon
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carbonyl-and-EWG2-Phosphonate-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R5 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
# Nucleophilic-Aliphatic-Substitution-Basic-Lg-Bromine-and-Nu-Azide
References:
[0]
Sn2
[1]
1.24: Nucleophilic Substitution, SN2, SN1 - Chemistry LibreTexts
[2]
Sn2
[3]
Reactions of Azides - Substitution, Reduction, Rearrangements, and More
Condition to enforce:
R1 = A-Aliphatic-Carbon
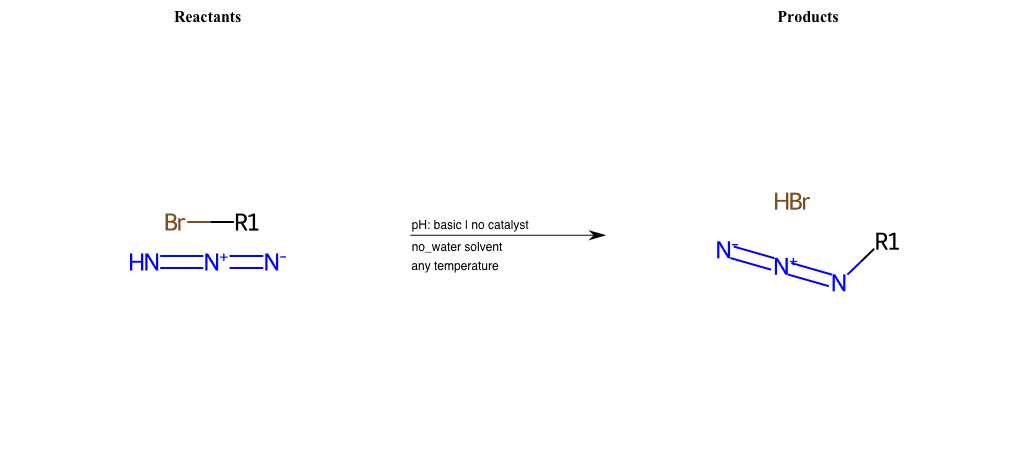
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carbonyl-and-EWG2-Nitrite-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
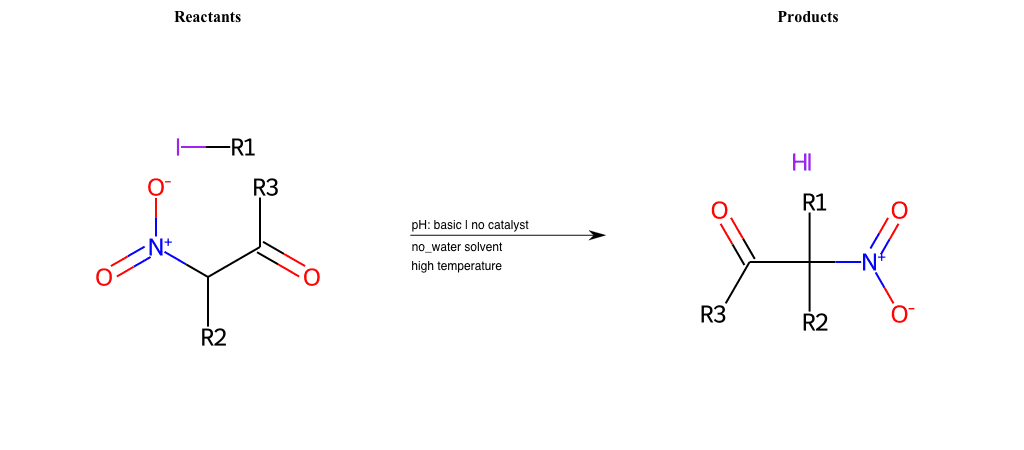
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carbonyl-and-EWG2-Carbonyl-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R4 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R5 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
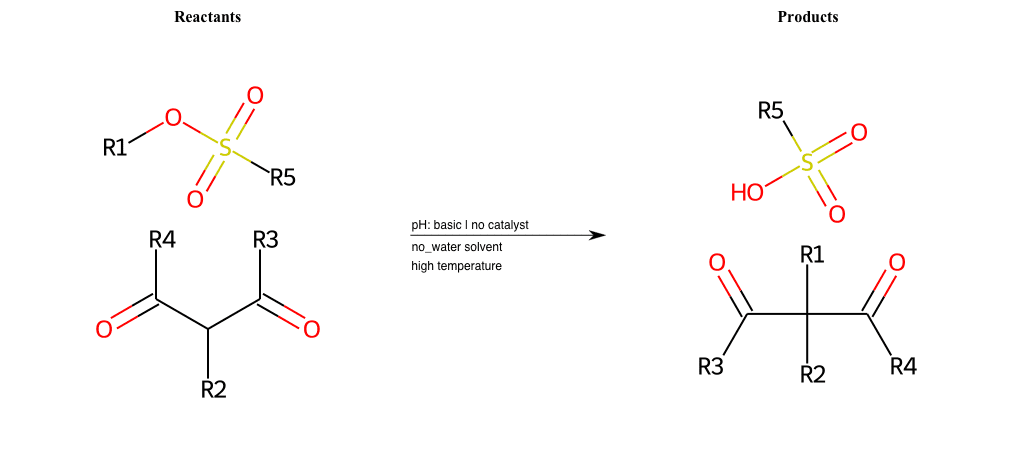
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Nitrile-and-EWG2-Nitrite-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
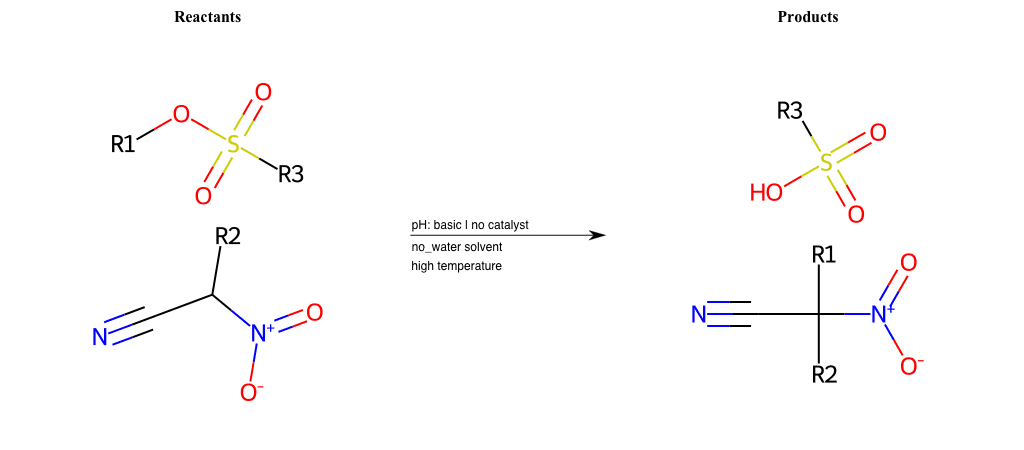
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Phosphonate-and-EWG2-Phosphonate-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R5 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R6 = A-Aliphatic-Carbon, A-Aromatic-Carbon
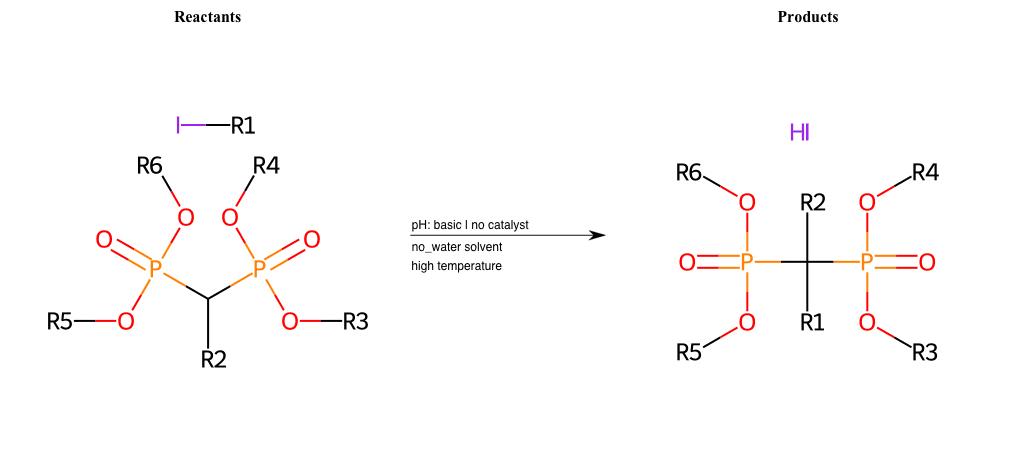
# Nucleophilic-Aliphatic-Substitution-Beta-acid-EWG1-Carboxyl-and-EWG2-Phosphonate-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = A-Aliphatic-Carbon
R2 = H, A-Aliphatic-Carbon
R3 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
R4 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R5 = A-Aliphatic-Carbon, A-Aromatic-Carbon
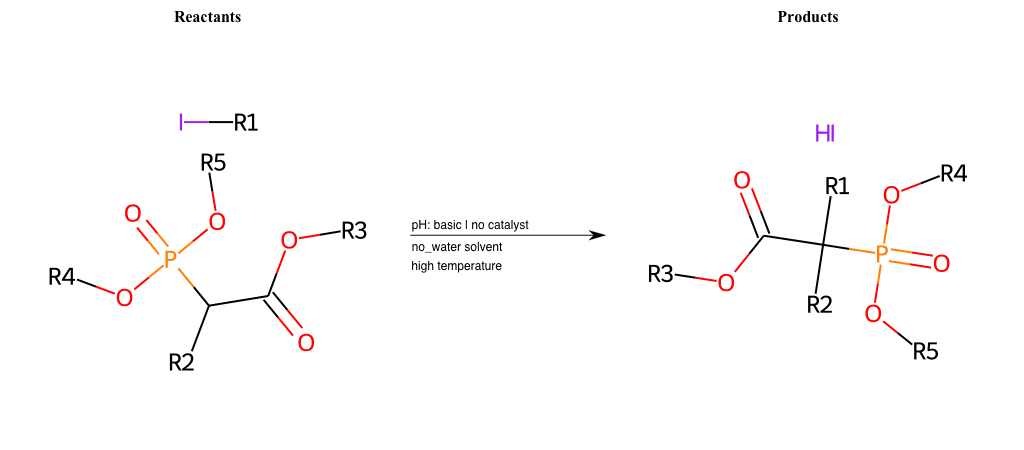
# Epoxide-Ring-Opening-Nu-Bromine-acid
References:
[0]
Epoxidation - Chemistry LibreTexts
[1]
Epoxides Ring-Opening Reactions - Chemistry Steps
Condition to enforce:
R1 = L-A, A-Aromatic-Carbon
R2 = L-A, A-Aromatic-Carbon
R3 = L-A, A-Aromatic-Carbon
R4 = L-A, A-Aromatic-Carbon