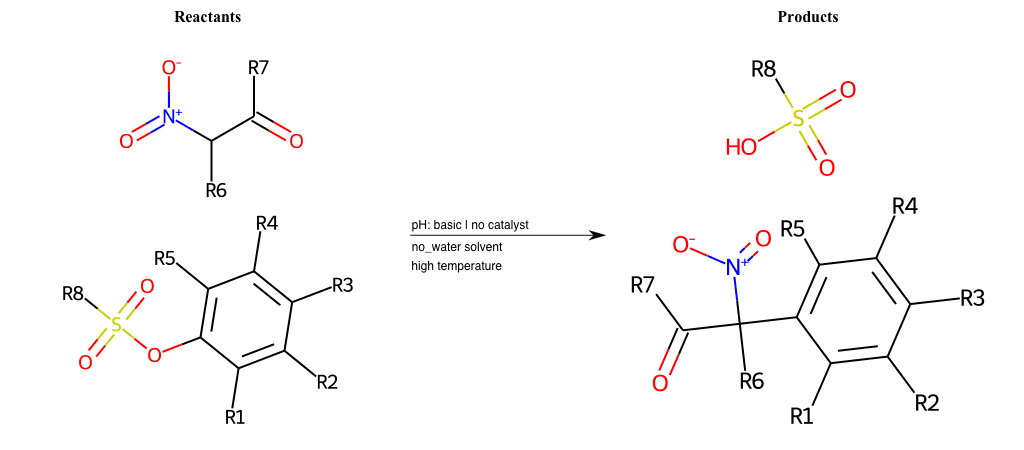
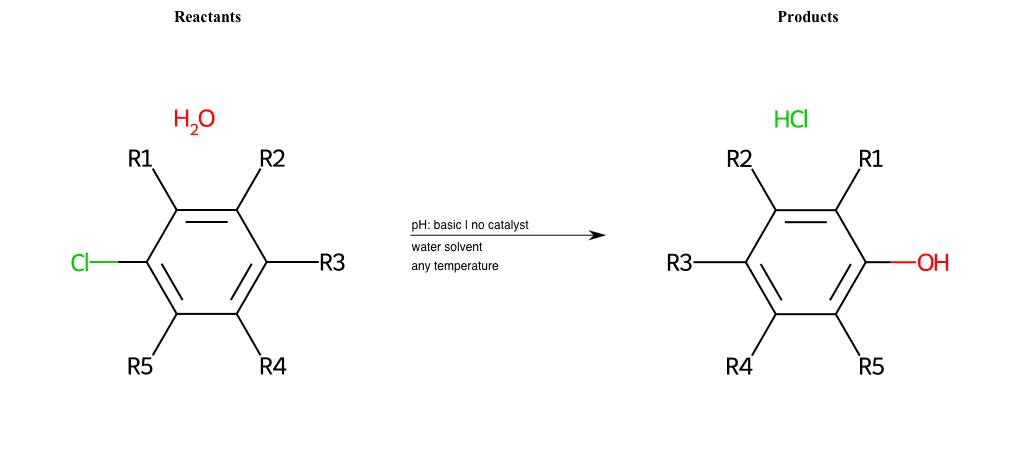
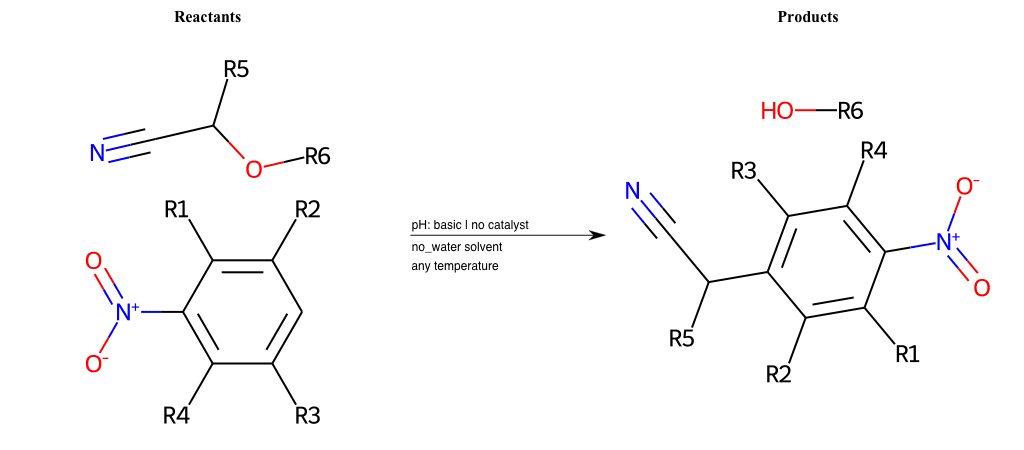
Nucleophilic-Aromatic-Substitutions
- A nucleophile (Nu-) attack an electron poor aromatic molecule, resulting in the substitution of a leaving group
- Works well with electron withdrawing groups (EWG), such as NO2, since it activates the ring
- The EWGs work well in the ortho or para positions since the Nu- puts a negative charge on the ring and the EWG can help stabilize it
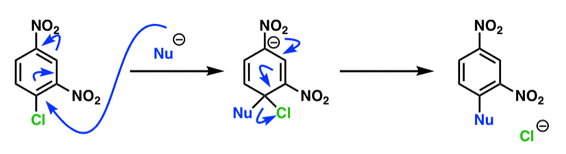
https://www.masterorganicchemistry.com/2018/08/20/nucleophilic-aromatic-substitution-nas/
https://en.wikipedia.org/wiki/Nucleophilic_aromatic_substitution
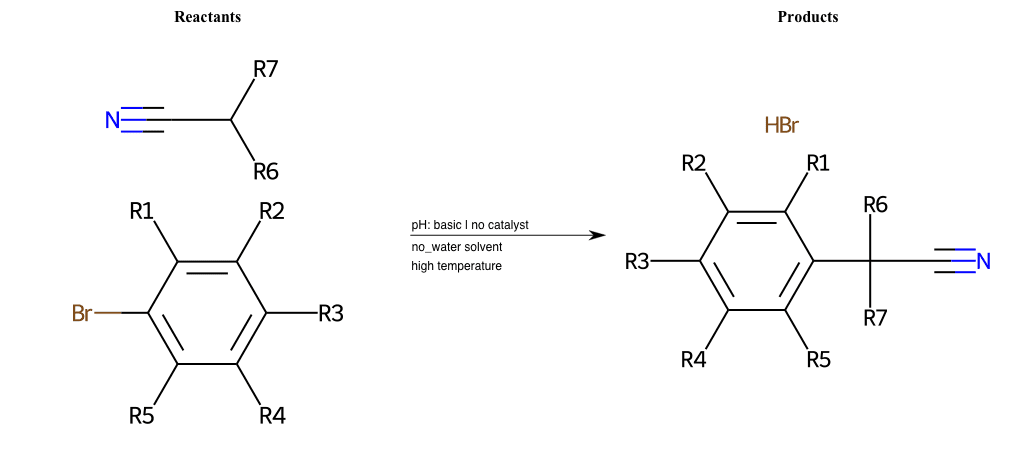
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Nitrile-and-EWG2-Alkane-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = H, A-Aliphatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
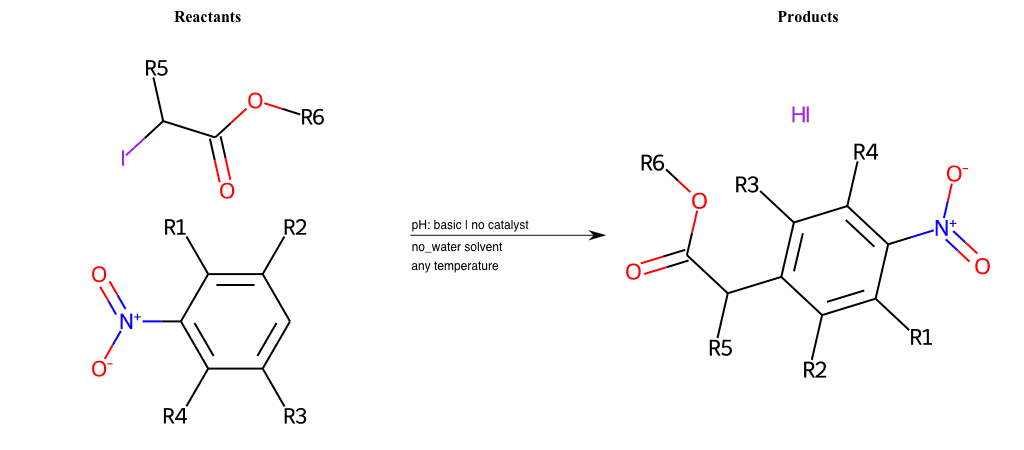
# Vicarious-Nucleophilic-Substitution-Ortho-X-Iodine-and-EWG-Carboxyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
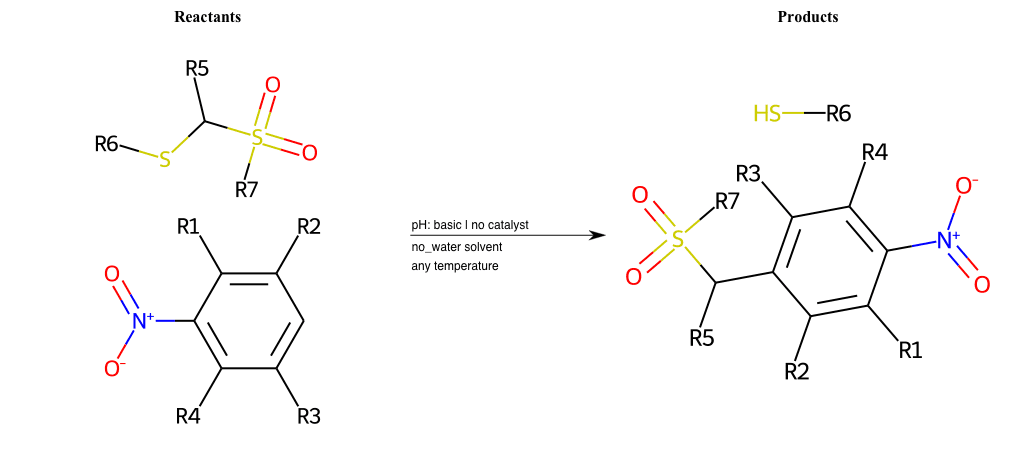
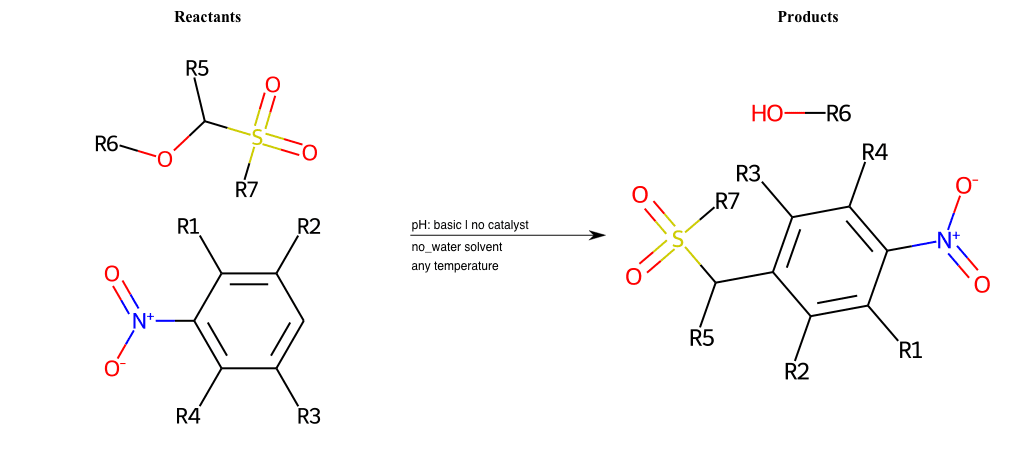
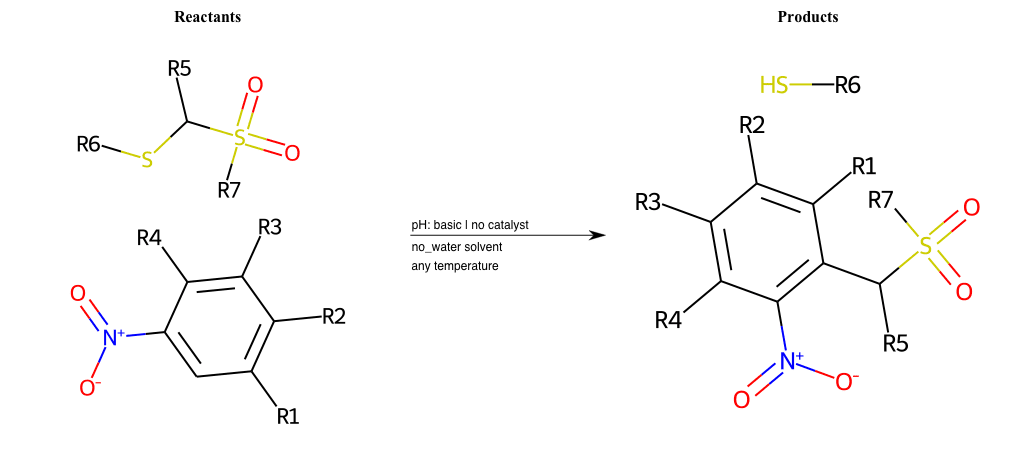
# Vicarious-Nucleophilic-Substitution-Ortho-X-Alkoxide-and-EWG-Sulfonyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R7 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
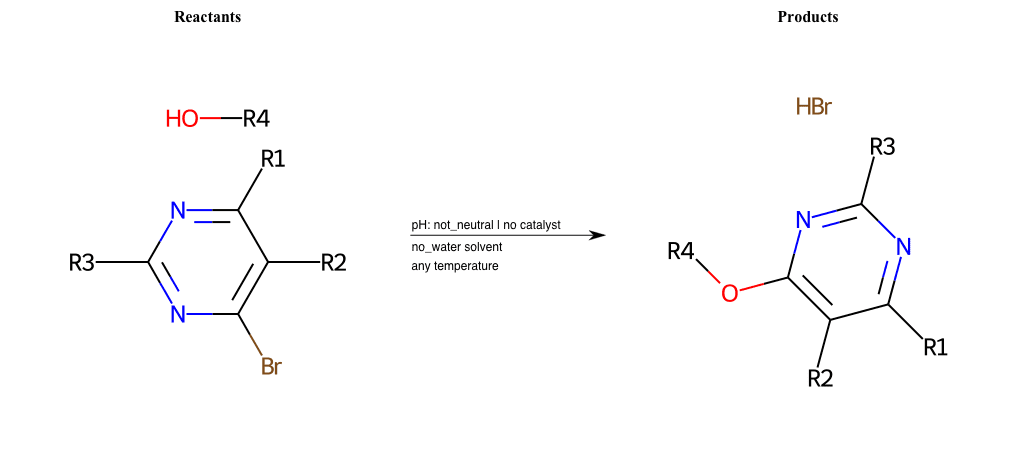
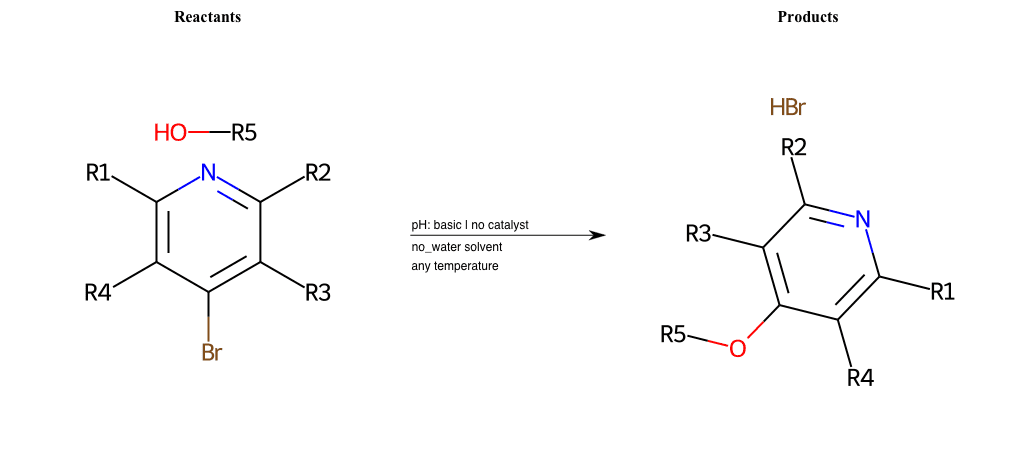
# Substituted-Pyridine-Nucleophilic-Aromatic-Substitution-Lg-Bromine-and-Nu-Alkoxide
References:
[0]
Weickgenannt_Jun_12.pdf
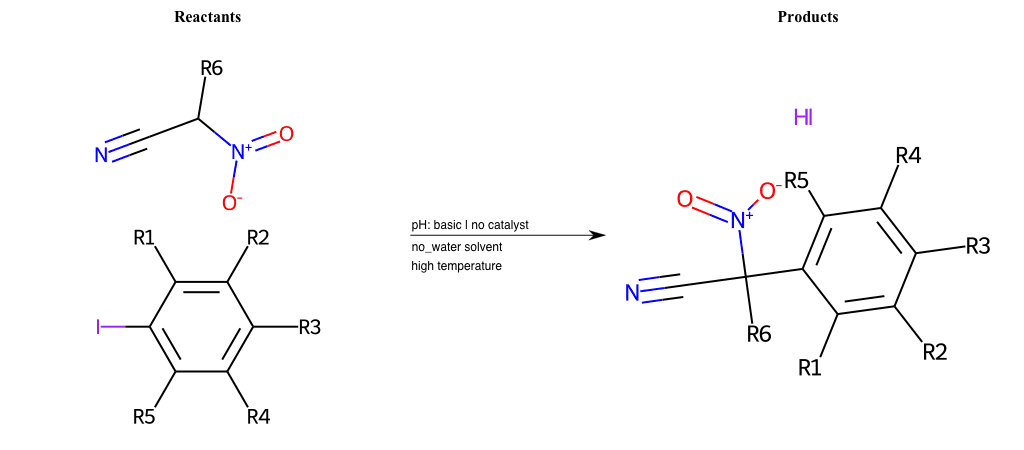
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Nitrile-and-EWG2-Nitrite-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
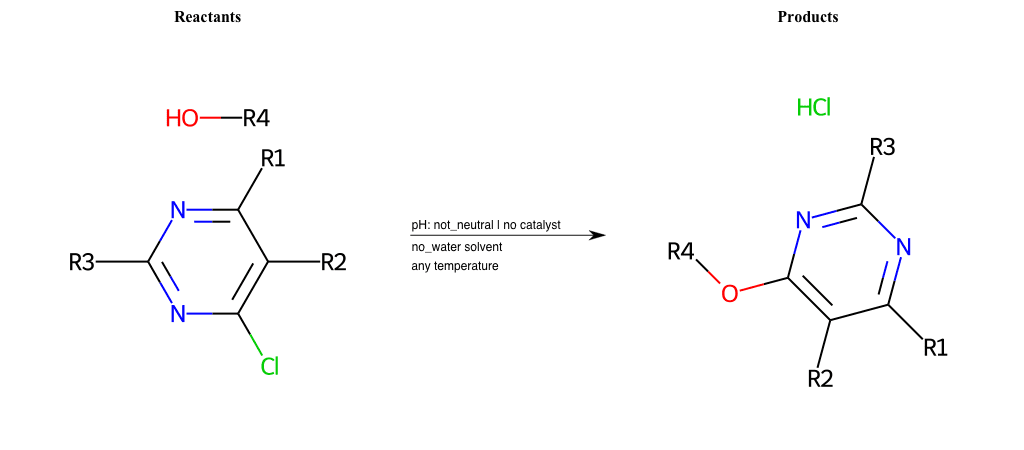
# Substituted-Pyridine-Nucleophilic-Aromatic-Substitution-Lg-Chlorine-and-Nu-Alkoxide
References:
[0]
Weickgenannt_Jun_12.pdf
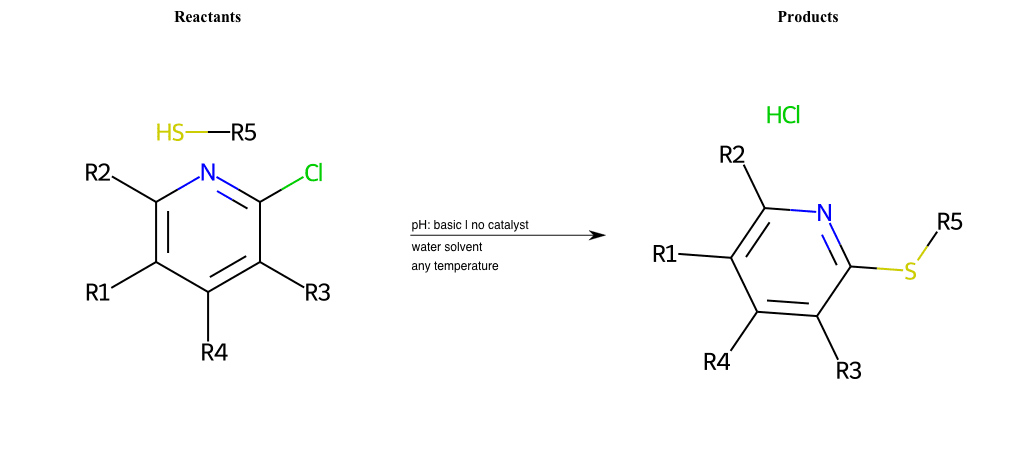
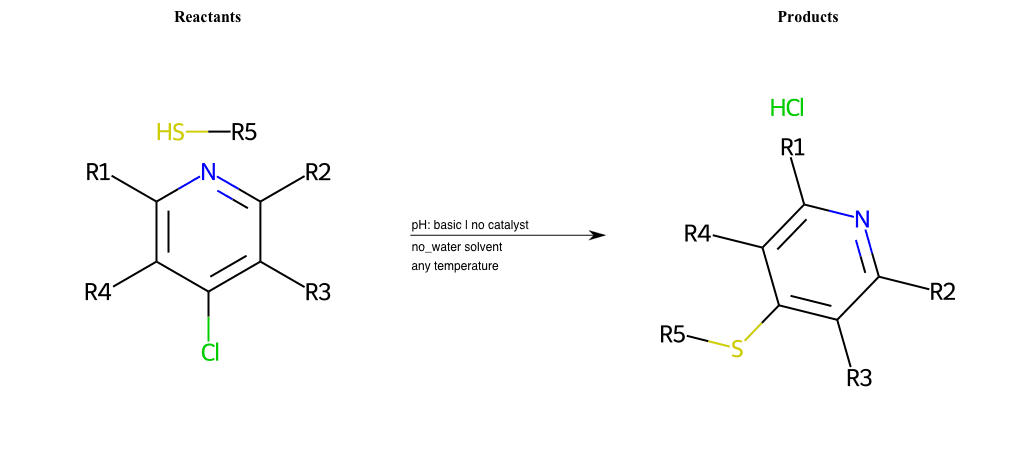
# Nucleophilic-Aromatic-Substitution-Pyridine-Ortho-Lg-Chlorine-and-Nu-Thiolate
References:
[0]
organic chemistry - Why does nucleophilic aromatic substitution occur at C-2 and C-4 of pyridine? - Chemistry Stack Exchange
[1]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[2]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[3]
Nucleophilic Aromatic Substitution - Chemistry Steps
[4]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
Condition to enforce:
R5 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
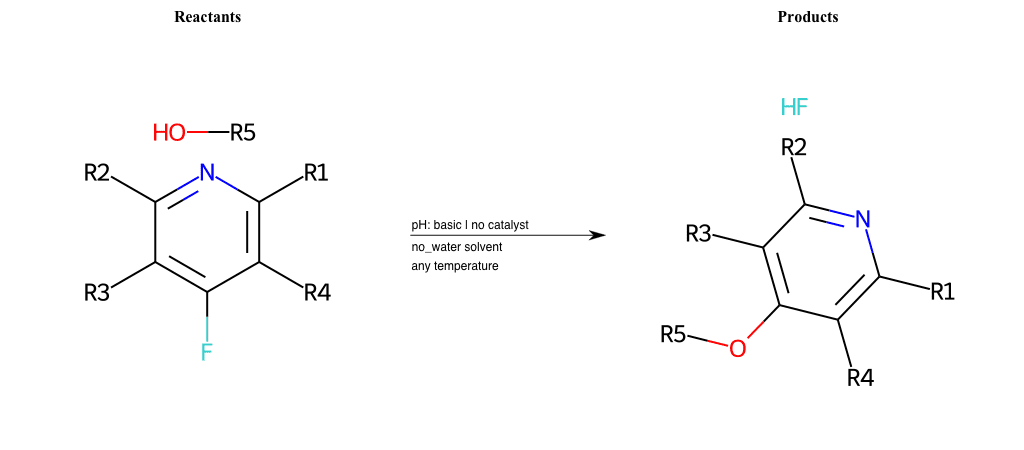
# Nucleophilic-Aromatic-Substitution-Pyridine-Para-Lg-Fluorine-and-Nu-Alkoxide
References:
[0]
organic chemistry - Why does nucleophilic aromatic substitution occur at C-2 and C-4 of pyridine? - Chemistry Stack Exchange
[1]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[2]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[3]
Nucleophilic Aromatic Substitution - Chemistry Steps
[4]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
Condition to enforce:
R5 = A-Aliphatic-Carbon, A-Aromatic-Carbon
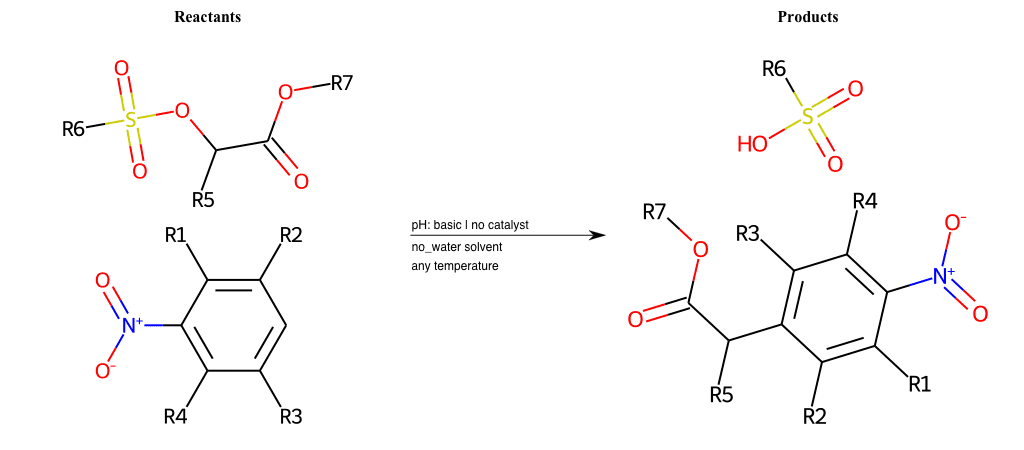
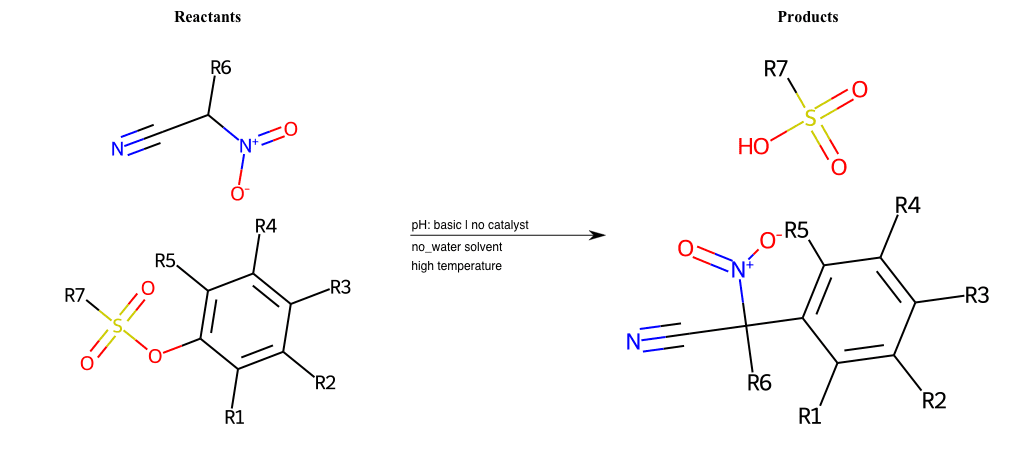
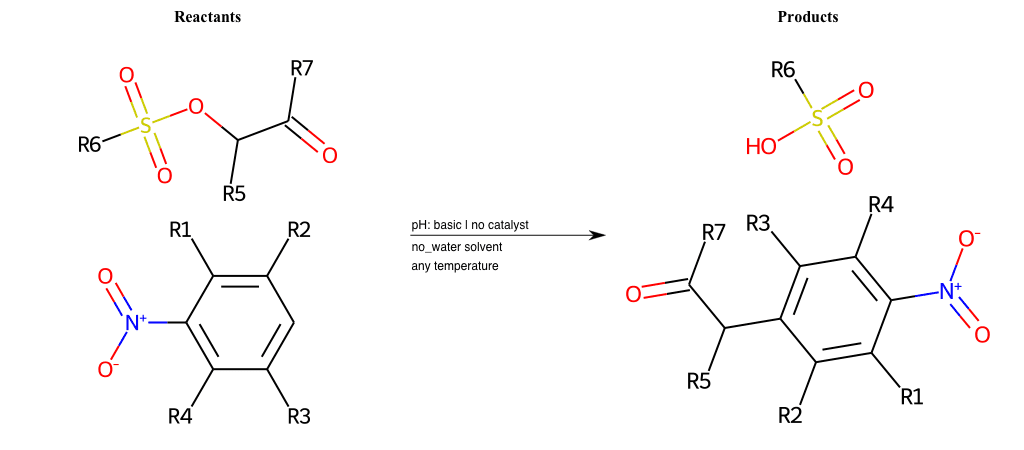
# Vicarious-Nucleophilic-Substitution-Ortho-X-Sulfonate-and-EWG-Carboxyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R7 = A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
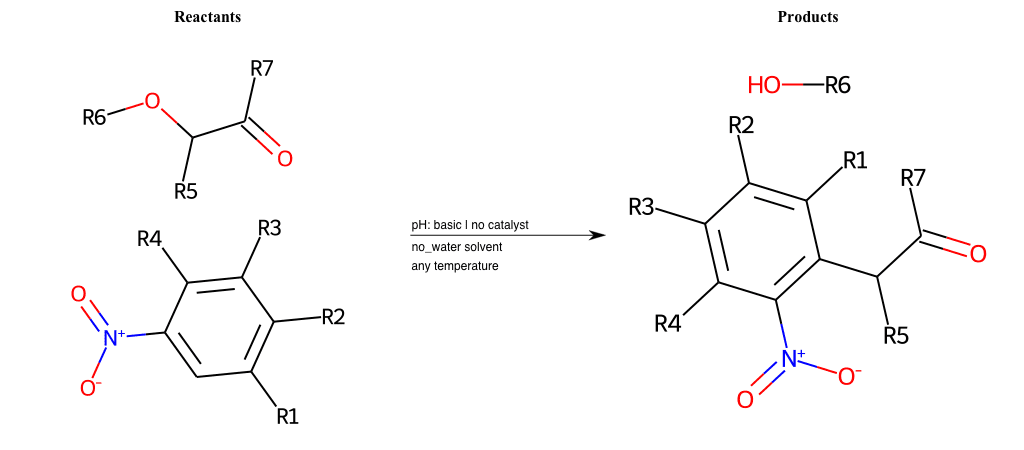
# Vicarious-Nucleophilic-Substitution-Para-X-Alkoxide-and-EWG-Carbonyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R7 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
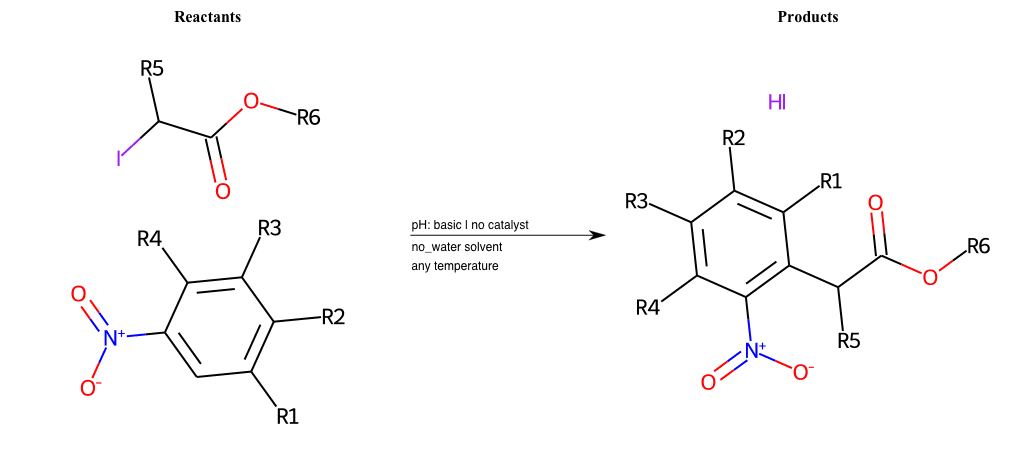
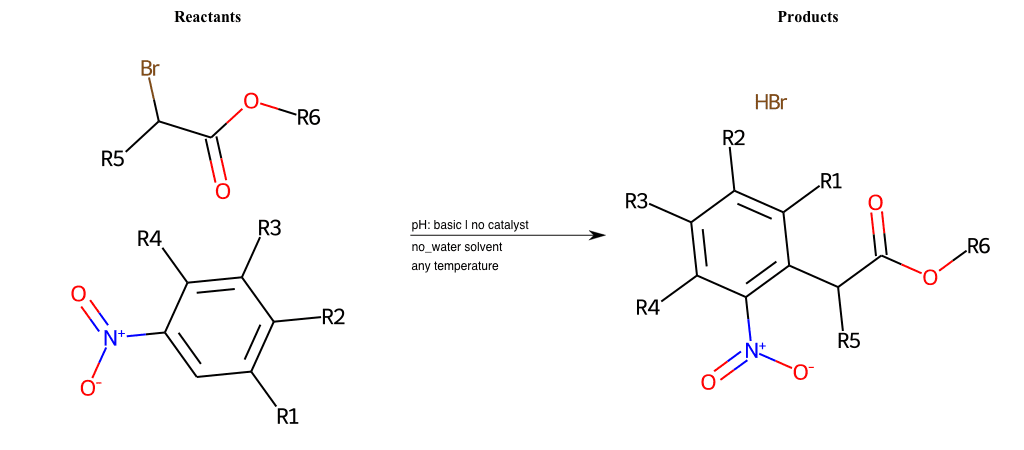
# Vicarious-Nucleophilic-Substitution-Para-X-Iodine-and-EWG-Carboxyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
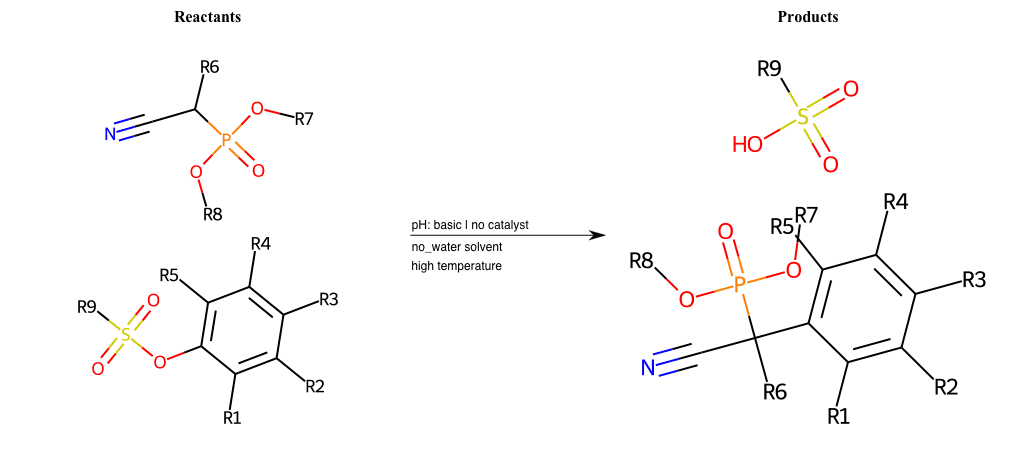
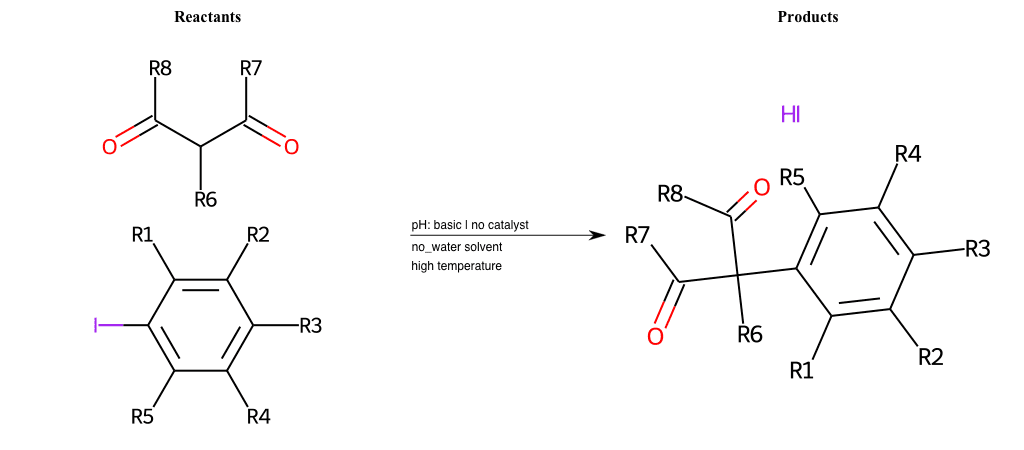
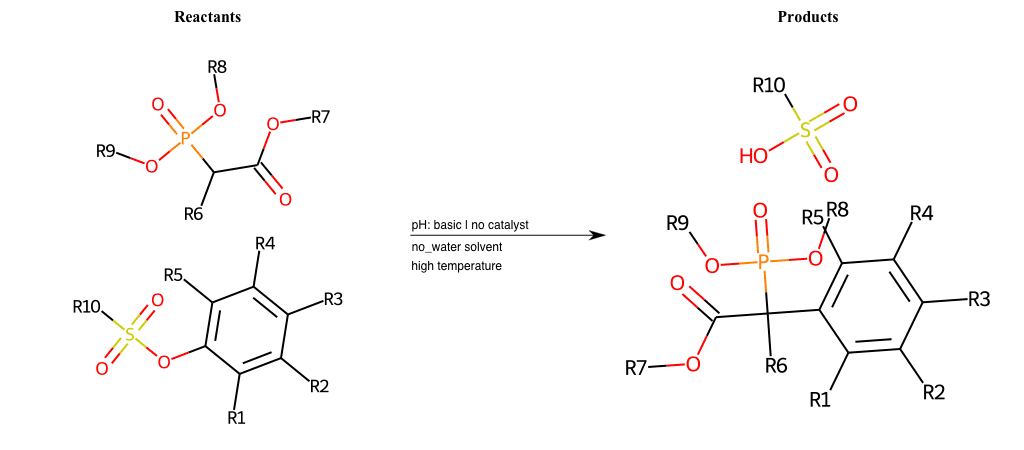
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Nitrile-and-EWG2-Phosphonate-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R8 = A-Aliphatic-Carbon, A-Aromatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
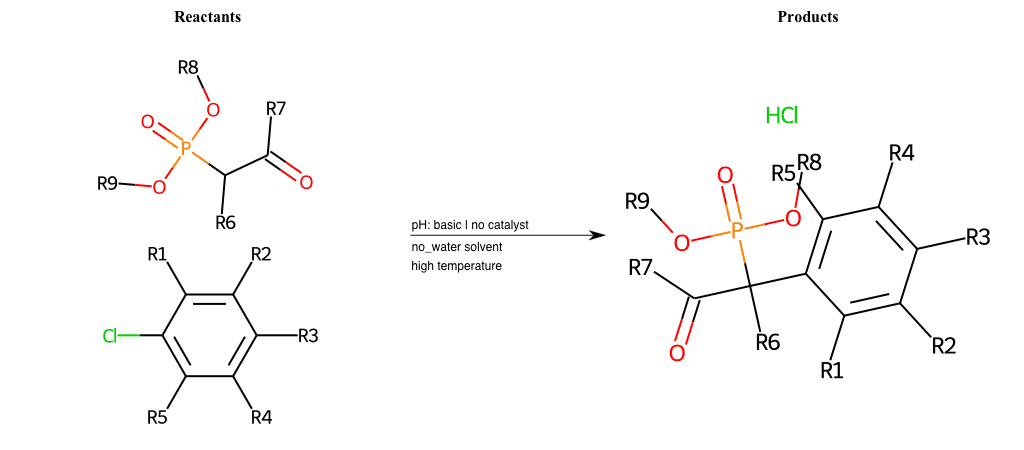
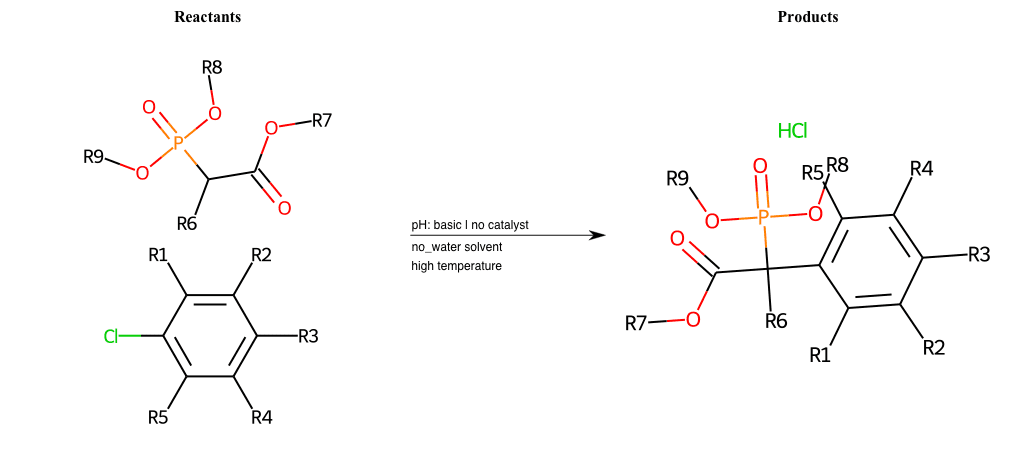
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Phosphonate-and-EWG2-Alkane-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R8 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R9 = H, A-Aliphatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
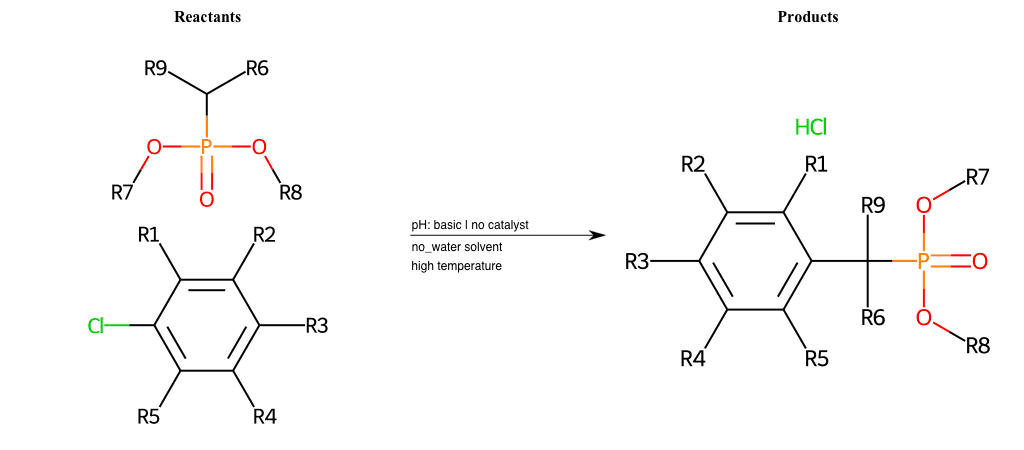
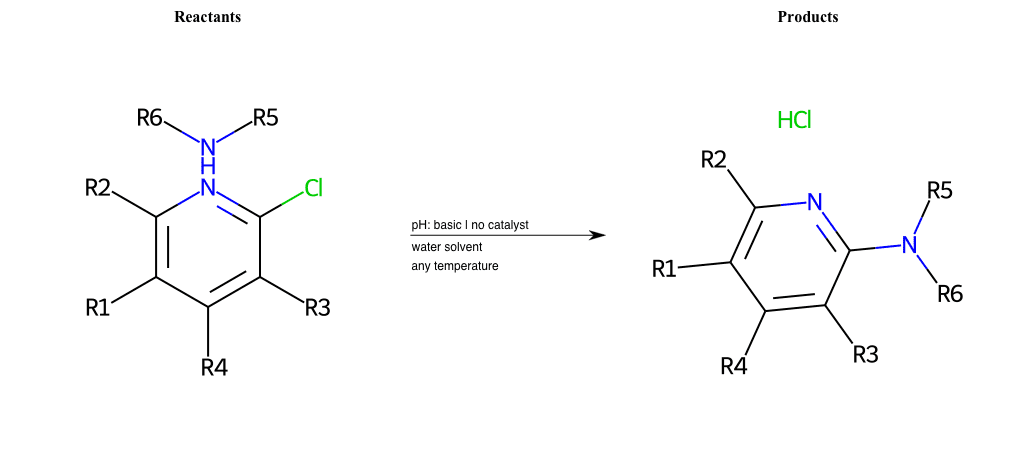
# Nucleophilic-Aromatic-Substitution-Pyridine-Ortho-Lg-Chlorine-and-Nu-Amino
References:
[0]
organic chemistry - Why does nucleophilic aromatic substitution occur at C-2 and C-4 of pyridine? - Chemistry Stack Exchange
[1]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[2]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[3]
Nucleophilic Aromatic Substitution - Chemistry Steps
[4]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
Condition to enforce:
R5 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
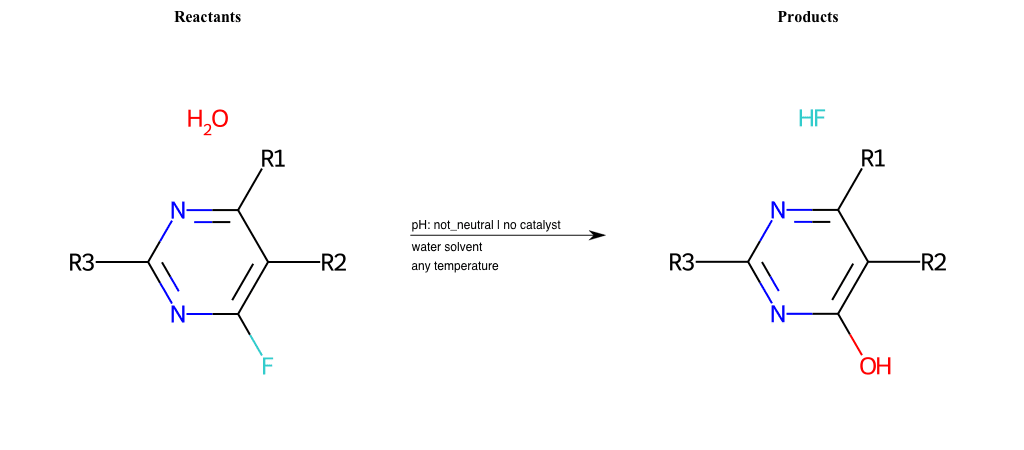
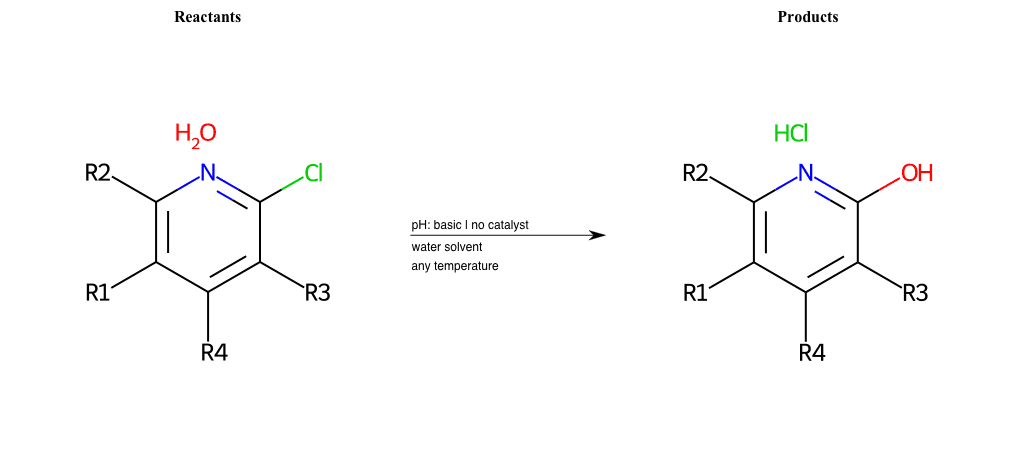
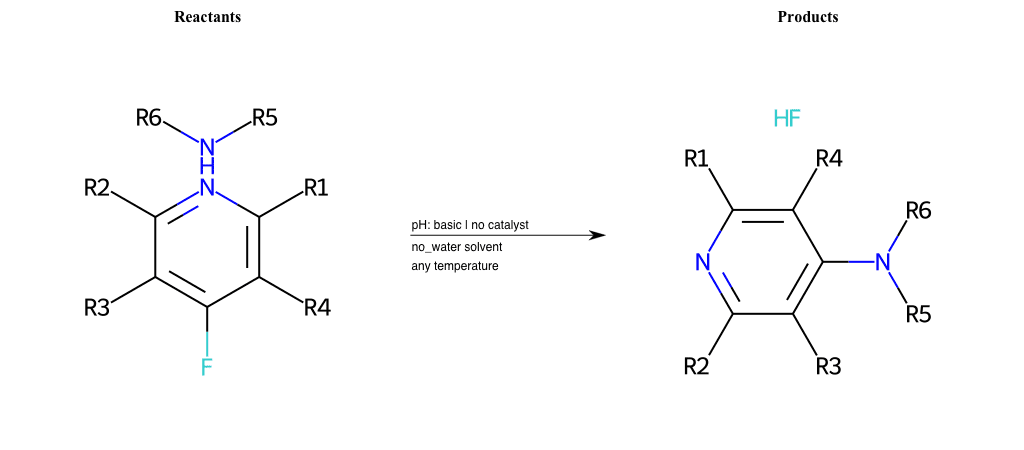
# Substituted-Pyridine-Nucleophilic-Aromatic-Substitution-Lg-Fluorine-and-Nu-Hydroxyl
References:
[0]
Weickgenannt_Jun_12.pdf
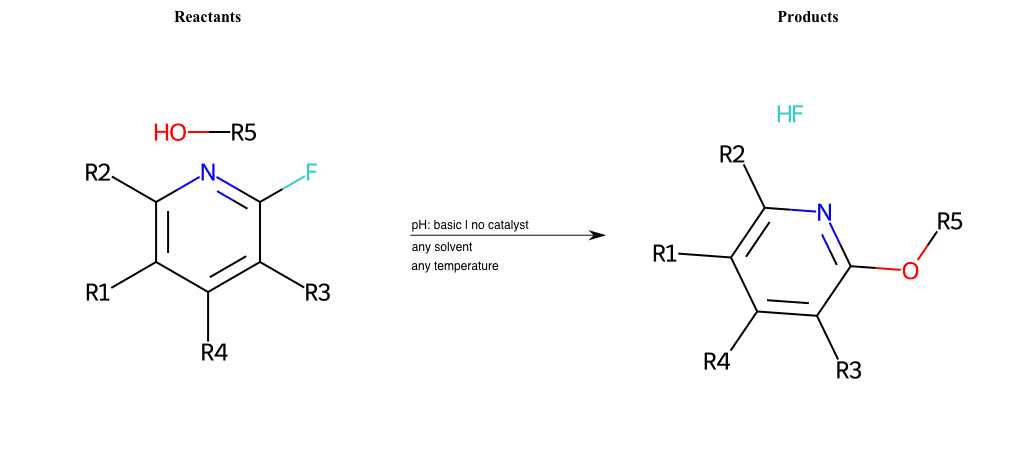
# Nucleophilic-Aromatic-Substitution-Pyridine-Ortho-Lg-Fluorine-and-Nu-Alkoxide
References:
[0]
organic chemistry - Why does nucleophilic aromatic substitution occur at C-2 and C-4 of pyridine? - Chemistry Stack Exchange
[1]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[2]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[3]
Nucleophilic Aromatic Substitution - Chemistry Steps
[4]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
Condition to enforce:
R5 = A-Aliphatic-Carbon, A-Aromatic-Carbon
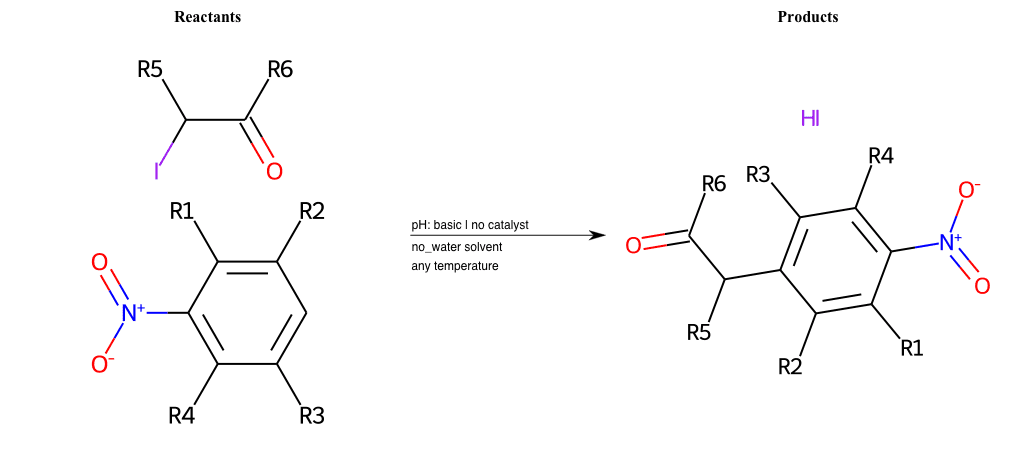
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carboxyl-and-EWG2-Nitrite-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
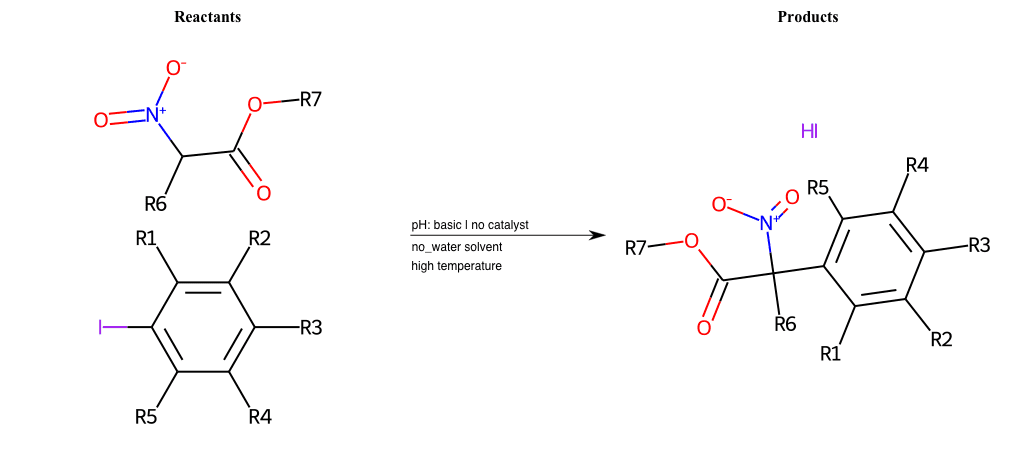
# Nucleophilic-Aromatic-Substitution-Pyridine-Para-Lg-Chlorine-and-Nu-Hydroxyl
References:
[0]
organic chemistry - Why does nucleophilic aromatic substitution occur at C-2 and C-4 of pyridine? - Chemistry Stack Exchange
[1]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[2]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[3]
Nucleophilic Aromatic Substitution - Chemistry Steps
[4]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
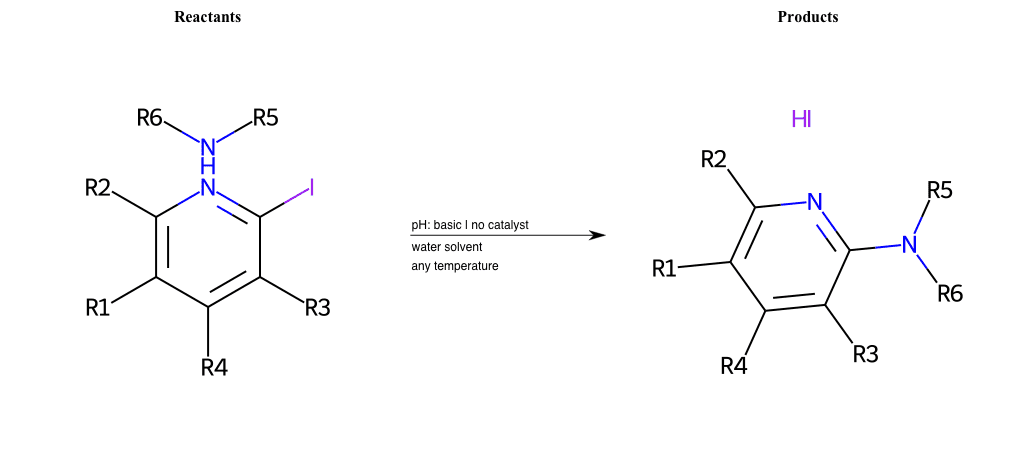
# Nucleophilic-Aromatic-Substitution-Pyridine-Ortho-Lg-Iodine-and-Nu-Amino
References:
[0]
organic chemistry - Why does nucleophilic aromatic substitution occur at C-2 and C-4 of pyridine? - Chemistry Stack Exchange
[1]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[2]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[3]
Nucleophilic Aromatic Substitution - Chemistry Steps
[4]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
Condition to enforce:
R5 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carbonyl-and-EWG2-Phosphonate-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
R8 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R9 = A-Aliphatic-Carbon, A-Aromatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
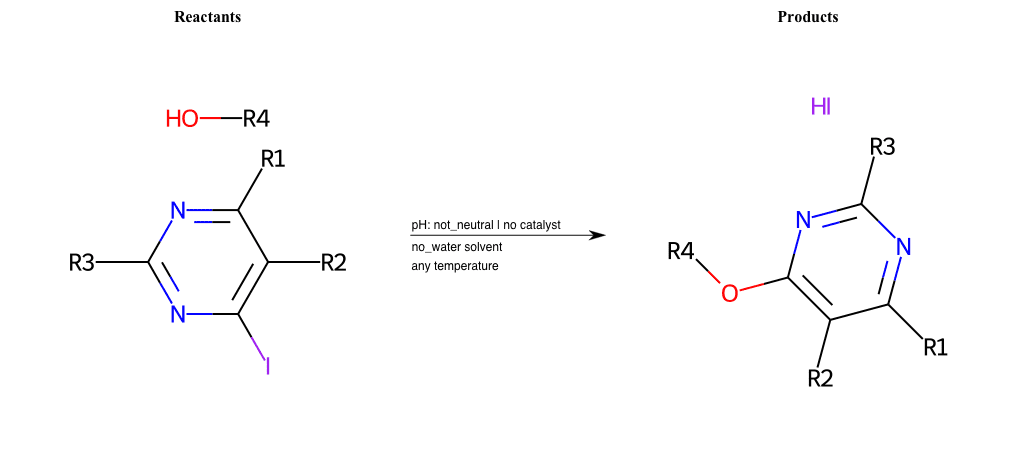
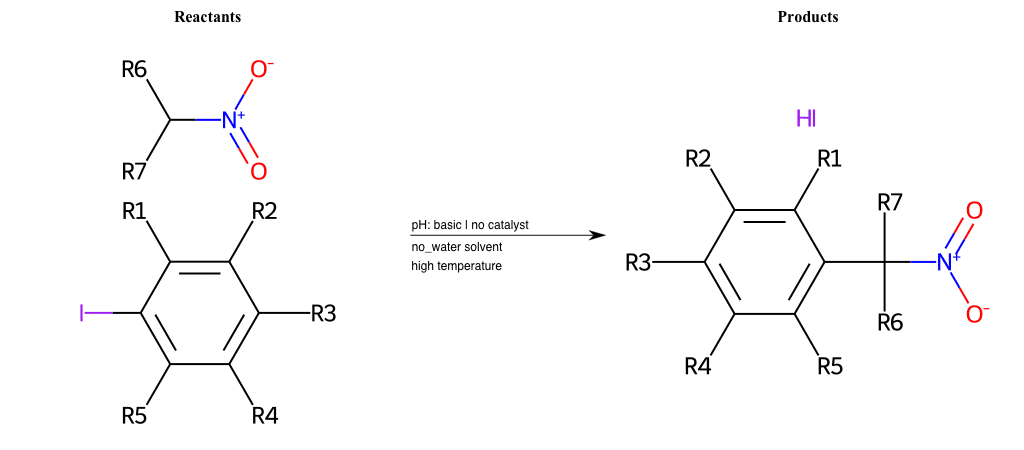
# Substituted-Pyridine-Nucleophilic-Aromatic-Substitution-Lg-Iodine-and-Nu-Alkoxide
References:
[0]
Weickgenannt_Jun_12.pdf
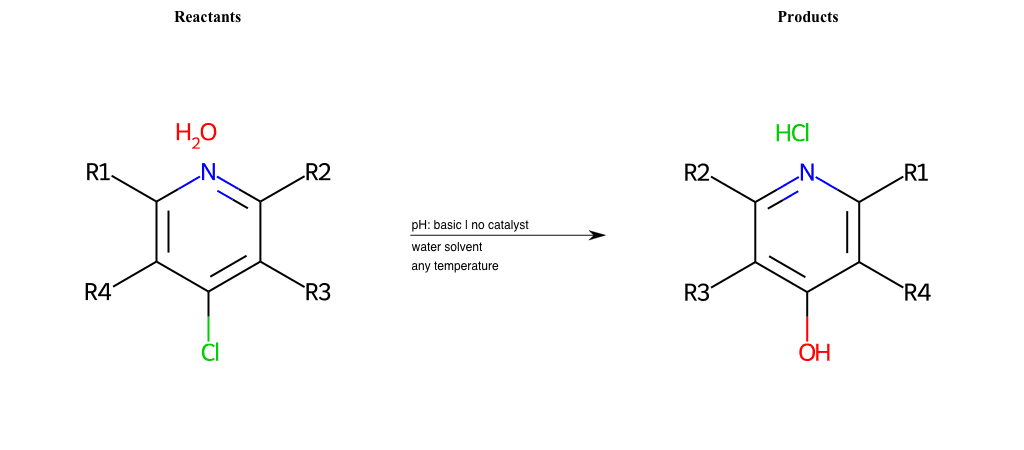
# Nucleophilic-Aromatic-Substitution-Pyridine-Ortho-Lg-Chlorine-and-Nu-Hydroxyl
References:
[0]
organic chemistry - Why does nucleophilic aromatic substitution occur at C-2 and C-4 of pyridine? - Chemistry Stack Exchange
[1]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[2]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[3]
Nucleophilic Aromatic Substitution - Chemistry Steps
[4]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
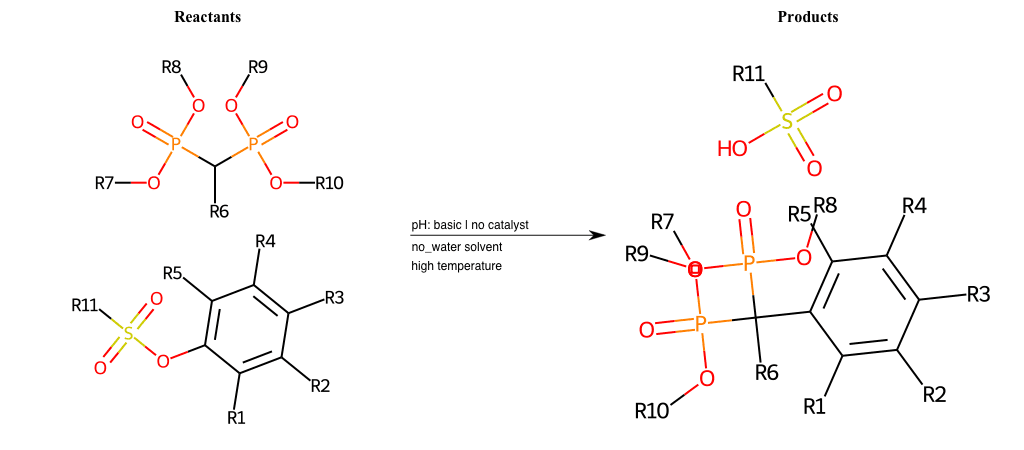
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Phosphonate-and-EWG2-Phosphonate-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R8 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R9 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R10 = A-Aliphatic-Carbon, A-Aromatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carbonyl-and-EWG2-Nitrite-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Nitrile-and-EWG2-Nitrite-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
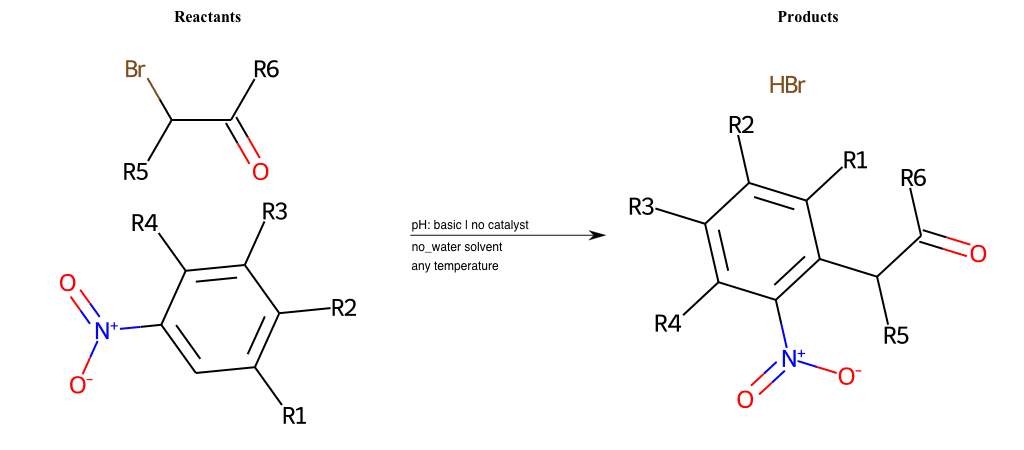
# Vicarious-Nucleophilic-Substitution-Para-X-Bromine-and-EWG-Carboxyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
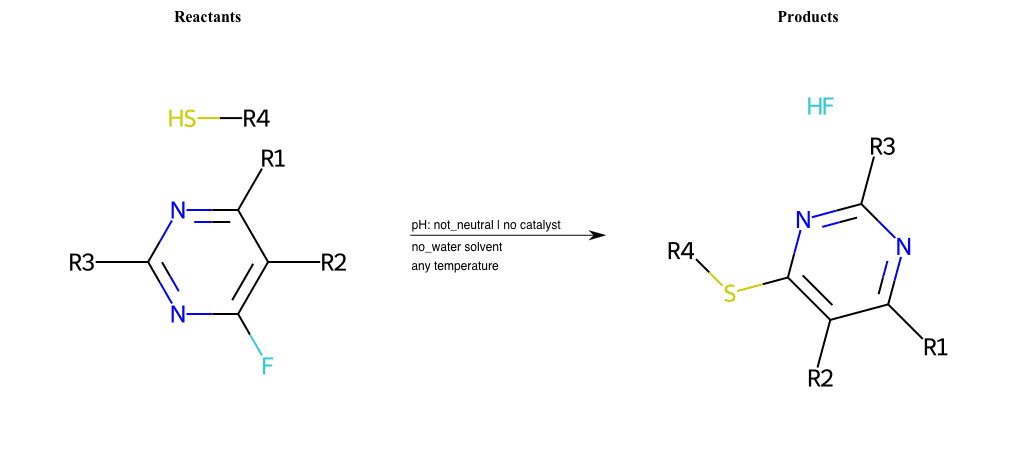
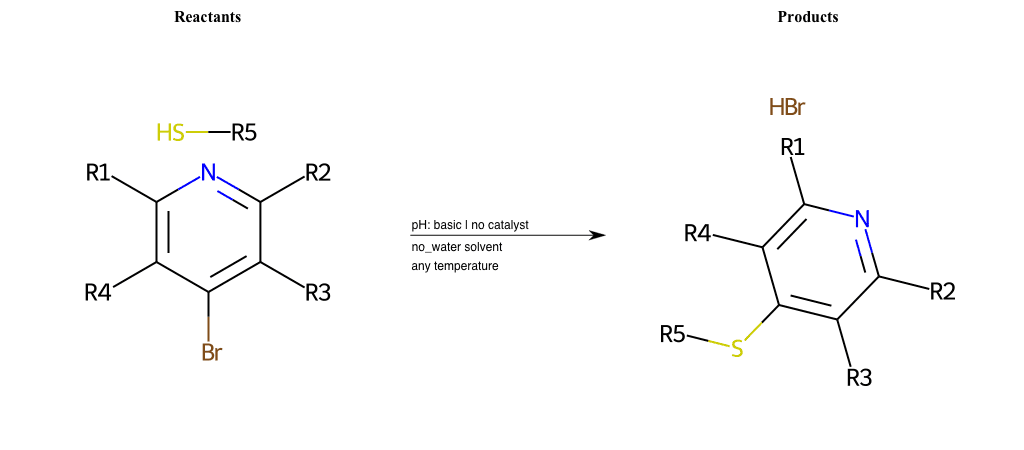
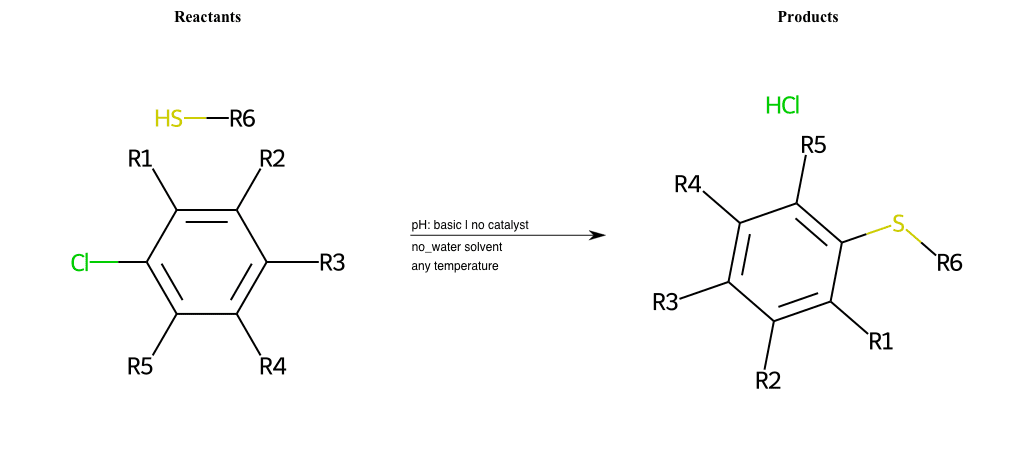
# Substituted-Pyridine-Nucleophilic-Aromatic-Substitution-Lg-Fluorine-and-Nu-Thiolate
References:
[0]
Weickgenannt_Jun_12.pdf
# Vicarious-Nucleophilic-Substitution-Ortho-X-Iodine-and-EWG-Carbonyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
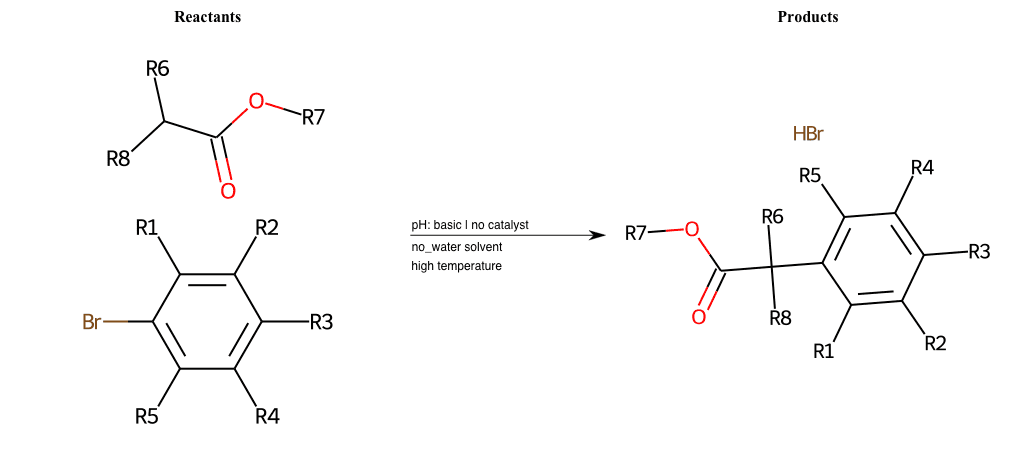
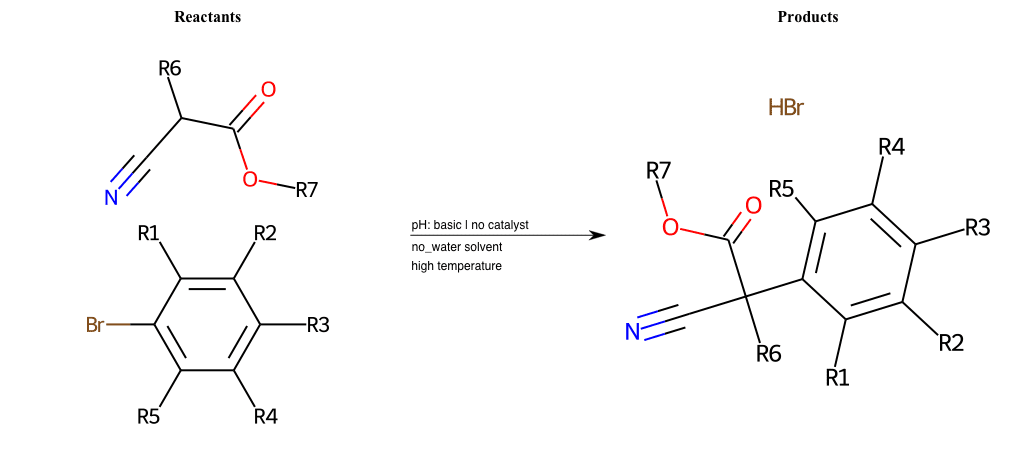
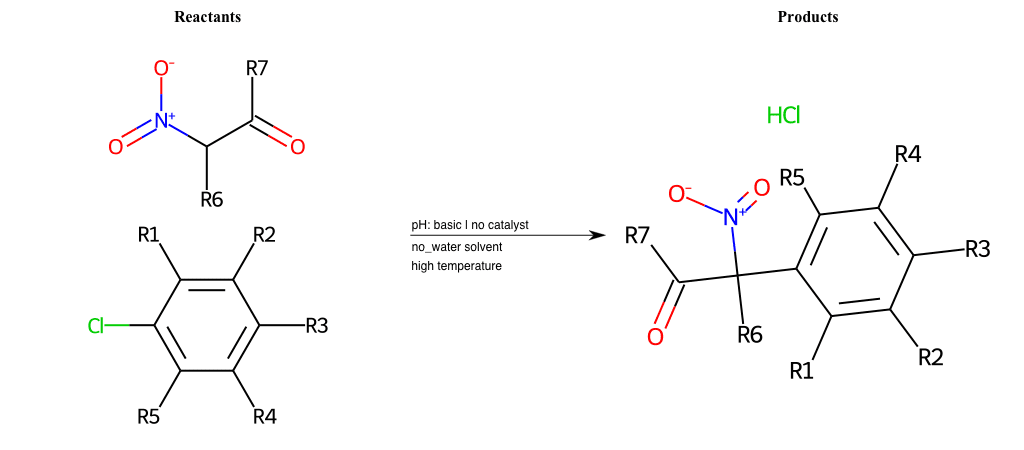
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carboxyl-and-EWG2-Alkane-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
R8 = H, A-Aliphatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
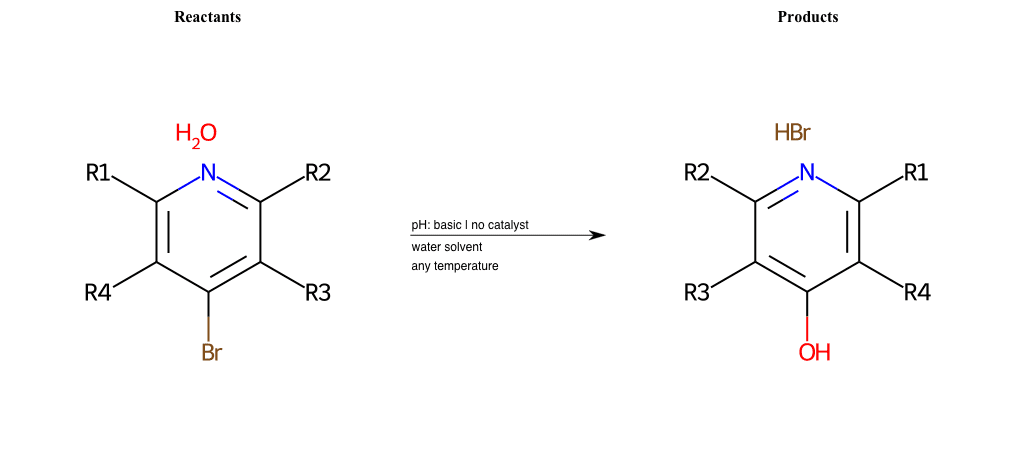
# Nucleophilic-Aromatic-Substitution-Pyridine-Para-Lg-Bromine-and-Nu-Hydroxyl
References:
[0]
organic chemistry - Why does nucleophilic aromatic substitution occur at C-2 and C-4 of pyridine? - Chemistry Stack Exchange
[1]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[2]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[3]
Nucleophilic Aromatic Substitution - Chemistry Steps
[4]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
# Vicarious-Nucleophilic-Substitution-Para-X-Bromine-and-EWG-Carbonyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Nitrite-and-EWG2-Alkane-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = H, A-Aliphatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
# Nucleophilic-Aromatic-Substitution-Pyridine-Para-Lg-Bromine-and-Nu-Thiolate
References:
[0]
organic chemistry - Why does nucleophilic aromatic substitution occur at C-2 and C-4 of pyridine? - Chemistry Stack Exchange
[1]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[2]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[3]
Nucleophilic Aromatic Substitution - Chemistry Steps
[4]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
Condition to enforce:
R5 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carbonyl-and-EWG2-Carbonyl-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
R8 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Nitrite-and-EWG2-Alkane-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = H, A-Aliphatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
# Nucleophilic-Aromatic-Substitution-Pyridine-Para-Lg-Iodine-and-Nu-Hydroxyl
References:
[0]
organic chemistry - Why does nucleophilic aromatic substitution occur at C-2 and C-4 of pyridine? - Chemistry Stack Exchange
[1]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[2]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[3]
Nucleophilic Aromatic Substitution - Chemistry Steps
[4]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carboxyl-and-EWG2-Phosphonate-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
R8 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R9 = A-Aliphatic-Carbon, A-Aromatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
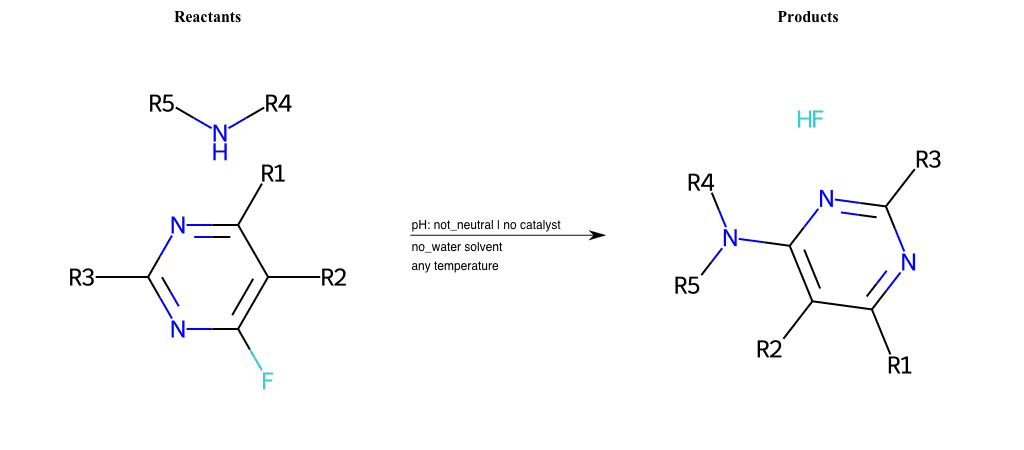
# Substituted-Pyridine-Nucleophilic-Aromatic-Substitution-Lg-Fluorine-and-Nu-Amino
References:
[0]
Weickgenannt_Jun_12.pdf
Condition to enforce:
R4 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
R5 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
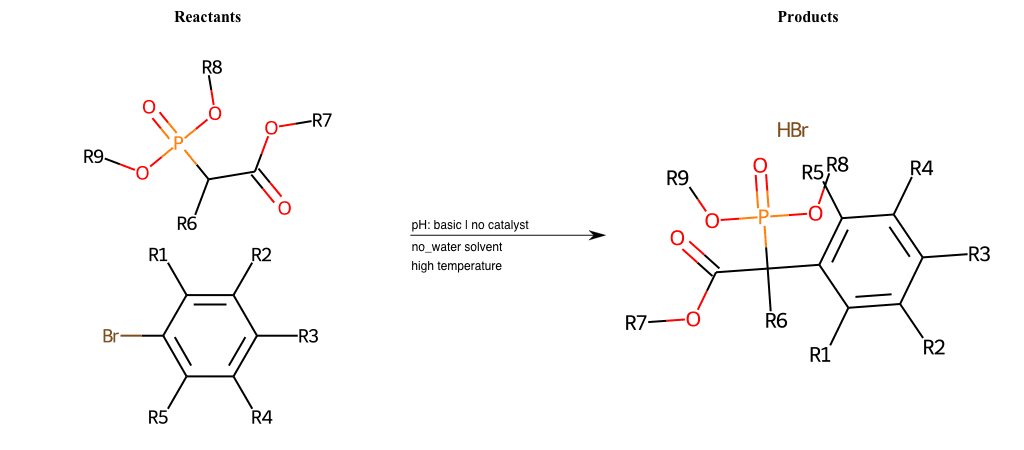
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carboxyl-and-EWG2-Phosphonate-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
R8 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R9 = A-Aliphatic-Carbon, A-Aromatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
# Nucleophilic-Aromatic-Substitution-Pyridine-Para-Lg-Iodine-and-Nu-Thiolate
References:
[0]
organic chemistry - Why does nucleophilic aromatic substitution occur at C-2 and C-4 of pyridine? - Chemistry Stack Exchange
[1]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[2]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[3]
Nucleophilic Aromatic Substitution - Chemistry Steps
[4]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
Condition to enforce:
R5 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carbonyl-and-EWG2-Carboxyl-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
R8 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
# Nucleophilic-Aromatic-Substitution-Pyridine-Para-Lg-Chlorine-and-Nu-Thiolate
References:
[0]
organic chemistry - Why does nucleophilic aromatic substitution occur at C-2 and C-4 of pyridine? - Chemistry Stack Exchange
[1]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[2]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[3]
Nucleophilic Aromatic Substitution - Chemistry Steps
[4]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
Condition to enforce:
R5 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
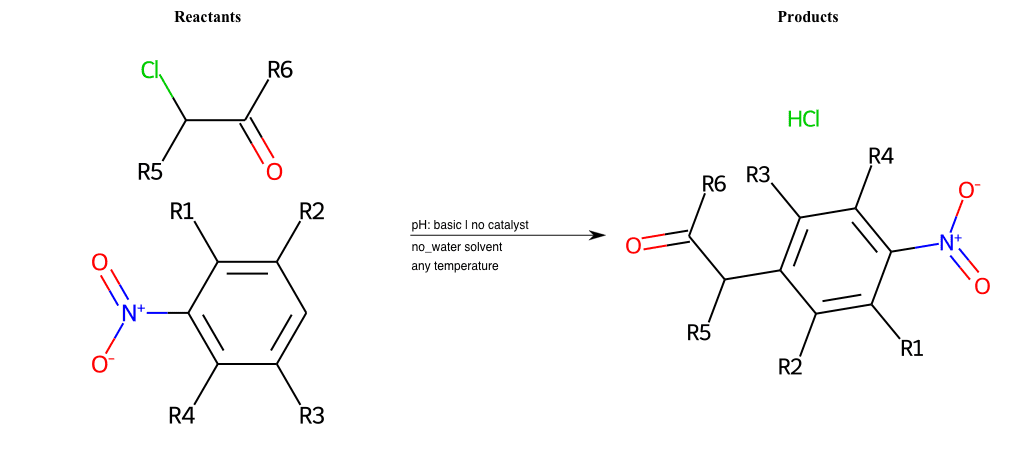
# Vicarious-Nucleophilic-Substitution-Ortho-X-Chlorine-and-EWG-Carbonyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
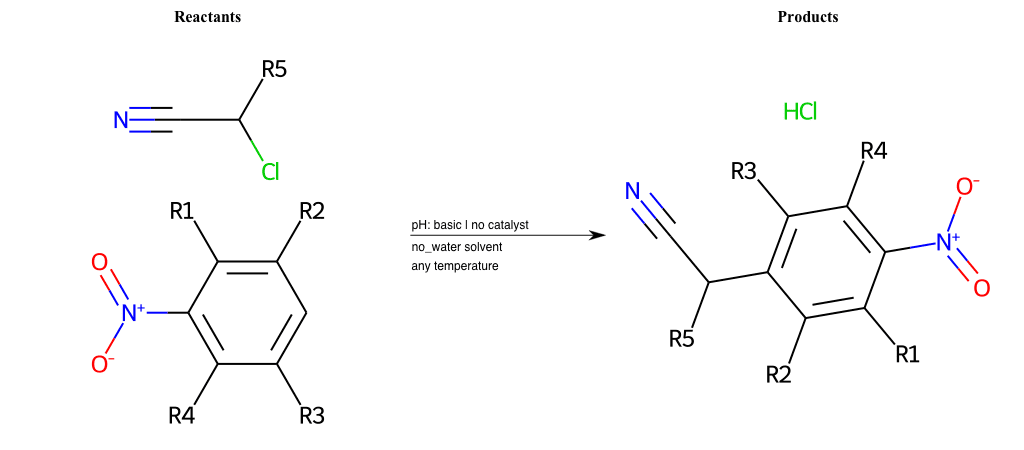
# Vicarious-Nucleophilic-Substitution-Ortho-X-Chlorine-and-EWG-Nitrile
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
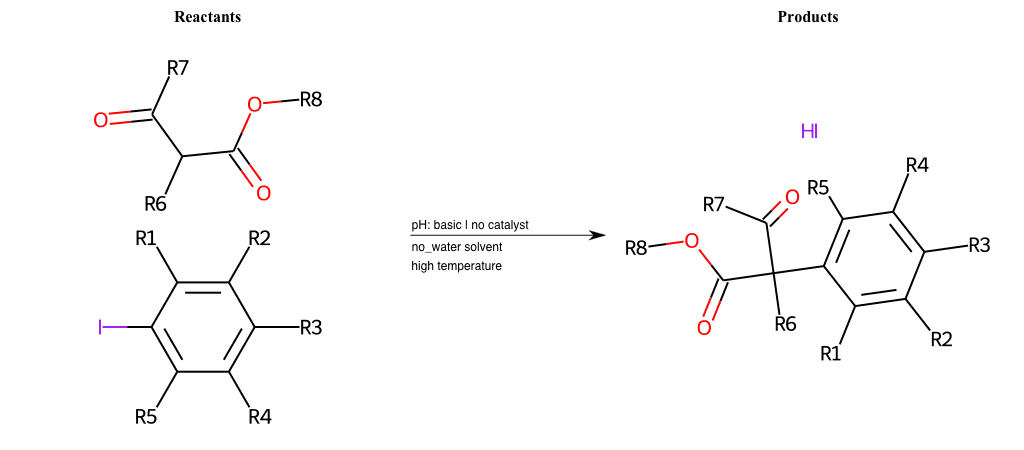
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carboxyl-and-EWG2-Phosphonate-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
R8 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R9 = A-Aliphatic-Carbon, A-Aromatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
# Vicarious-Nucleophilic-Substitution-Para-X-Alkoxide-and-EWG-Carboxyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R7 = A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
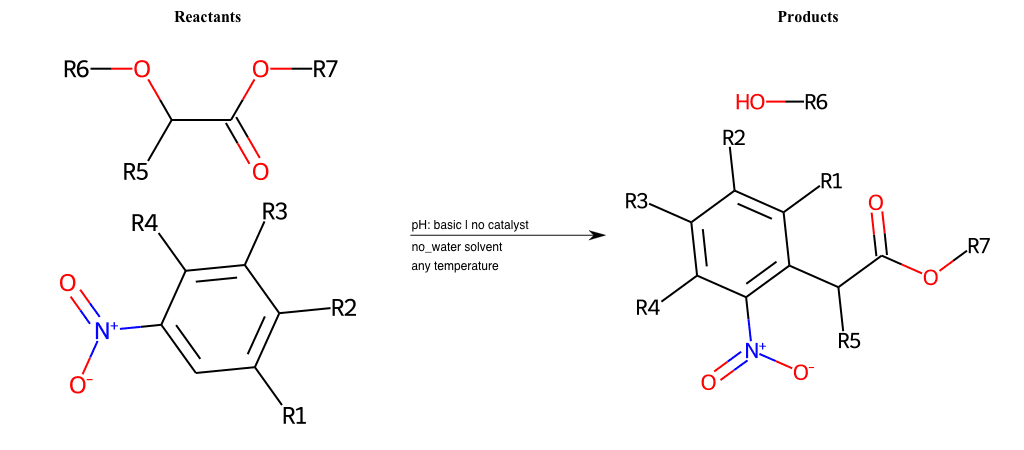
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Nitrite-and-EWG2-Nitrite-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carbonyl-and-EWG2-Carboxyl-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
R8 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
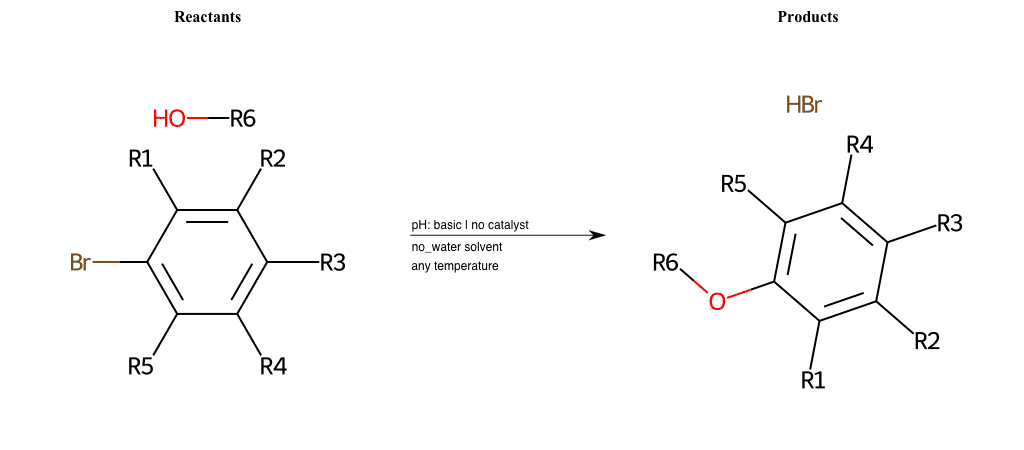
# Nucleophilic-Aromatic-Substitution-Lg-Bromine-and-Nu-Alkoxide
References:
[0]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[1]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[2]
Nucleophilic Aromatic Substitution - Chemistry Steps
[3]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
[4]
Explain why the trifluoromethyl (CF_3) group is meta directing in electrophilic aromatic substitution. What would you expect CF_3 to be activating or deactivating? Why? Can some one PLEASE help me!!!!! Thanks? | Socratic
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = A-Aliphatic-Carbon, A-Aromatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
# Vicarious-Nucleophilic-Substitution-Ortho-X-Sulfonate-and-EWG-Carbonyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R7 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carboxyl-and-EWG2-Nitrile-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
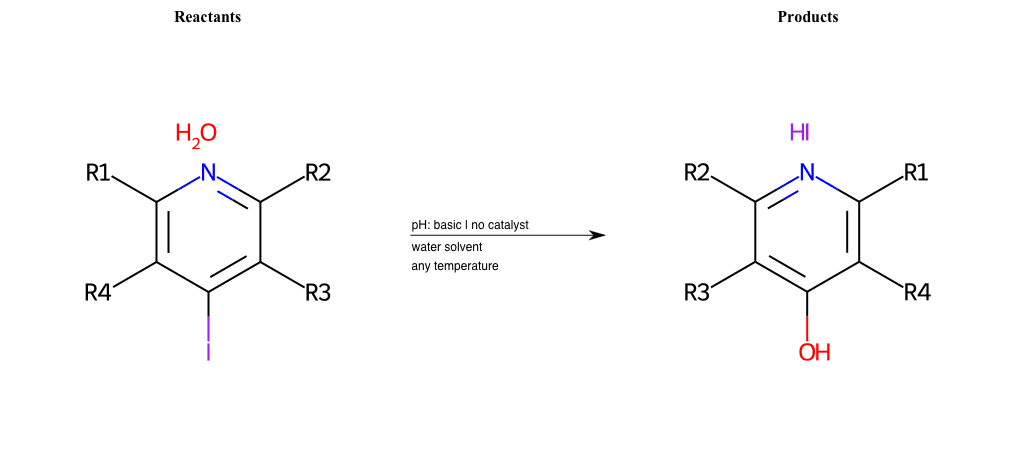
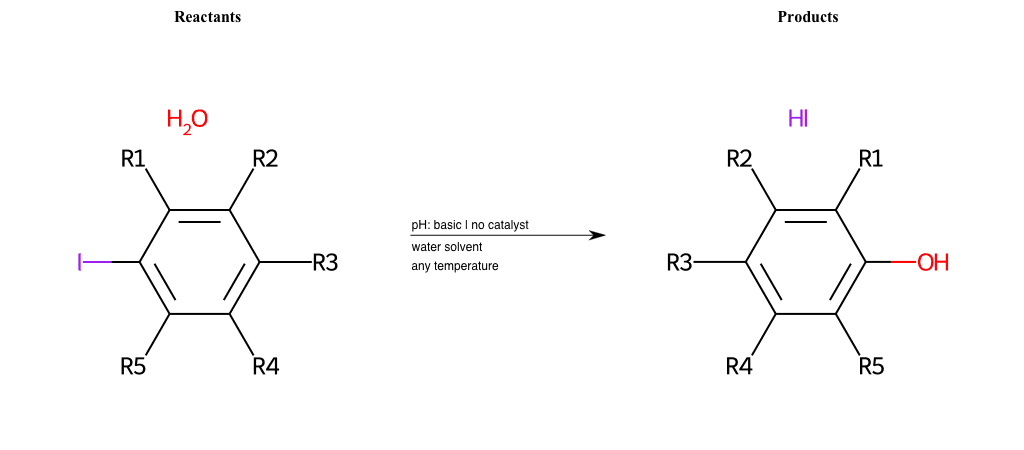
# Nucleophilic-Aromatic-Substitution-Lg-Iodine-and-Nu-Hydroxyl
References:
[0]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[1]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[2]
Nucleophilic Aromatic Substitution - Chemistry Steps
[3]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
[4]
Explain why the trifluoromethyl (CF_3) group is meta directing in electrophilic aromatic substitution. What would you expect CF_3 to be activating or deactivating? Why? Can some one PLEASE help me!!!!! Thanks? | Socratic
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
# Vicarious-Nucleophilic-Substitution-Para-X-Iodine-and-EWG-Carbonyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
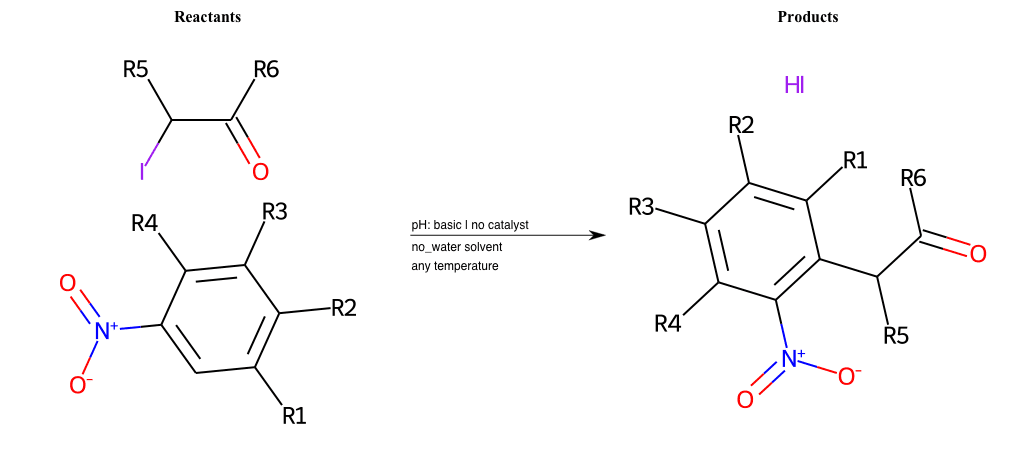
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Nitrite-and-EWG2-Alkane-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = H, A-Aliphatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
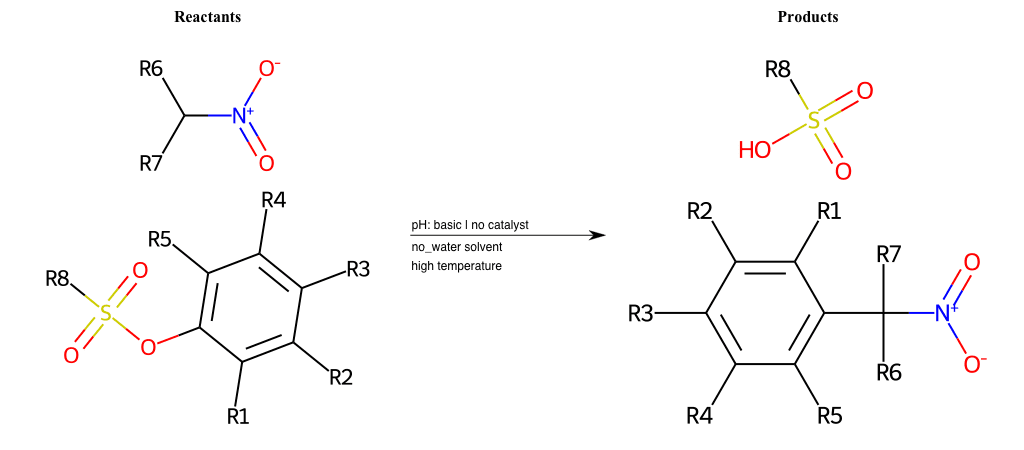
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Nitrile-and-EWG2-Nitrile-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
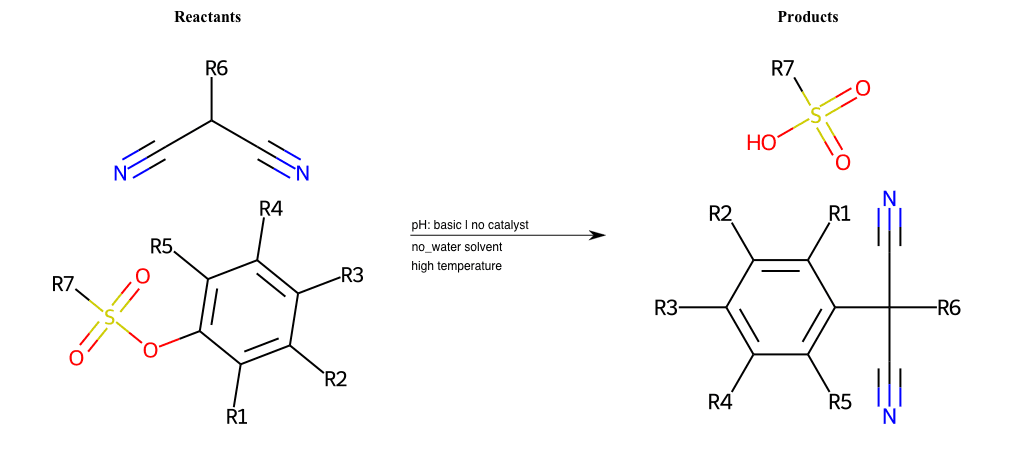
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carboxyl-and-EWG2-Nitrite-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
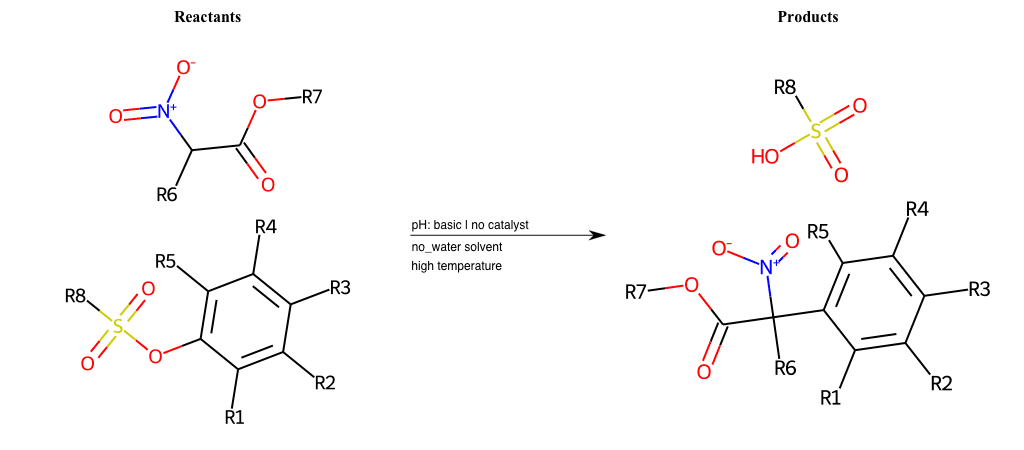
# Vicarious-Nucleophilic-Substitution-Para-X-Chlorine-and-EWG-Sulfonyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
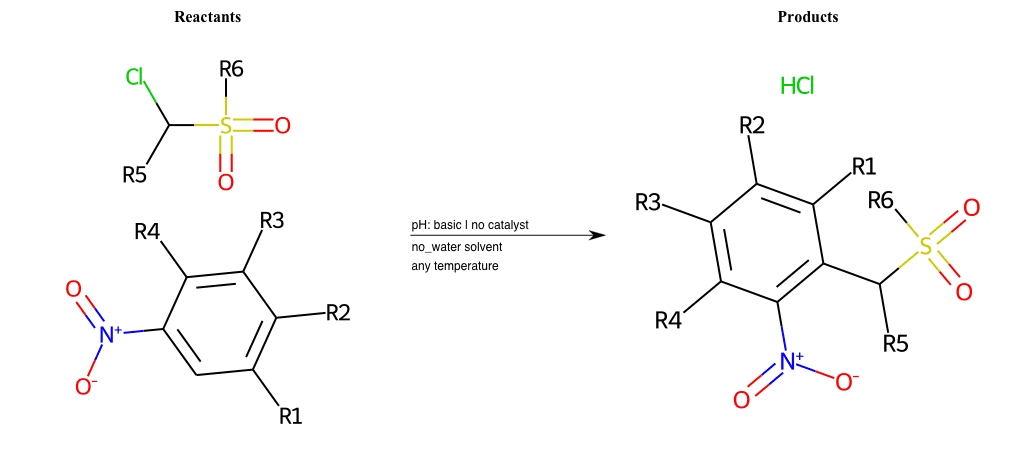
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Phosphonate-and-EWG2-Alkane-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R8 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R9 = H, A-Aliphatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
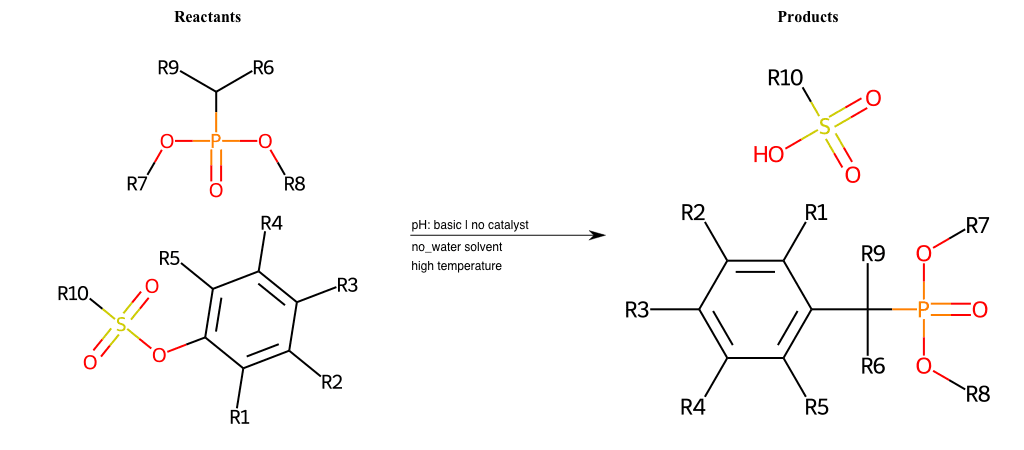
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carbonyl-and-EWG2-Nitrile-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
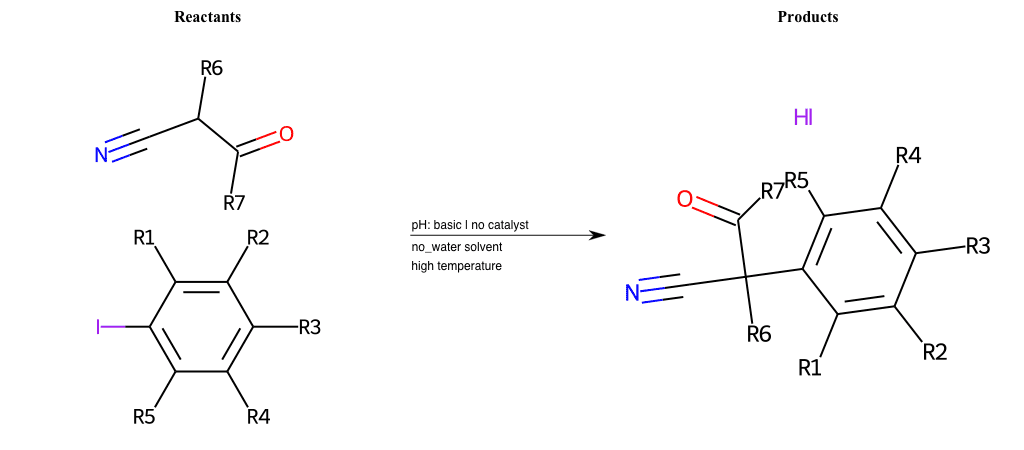
# Nucleophilic-Aromatic-Substitution-Nitric-Acid-Lg-Chlorine
References:
[0]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[1]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[2]
Nucleophilic Aromatic Substitution - Chemistry Steps
[3]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
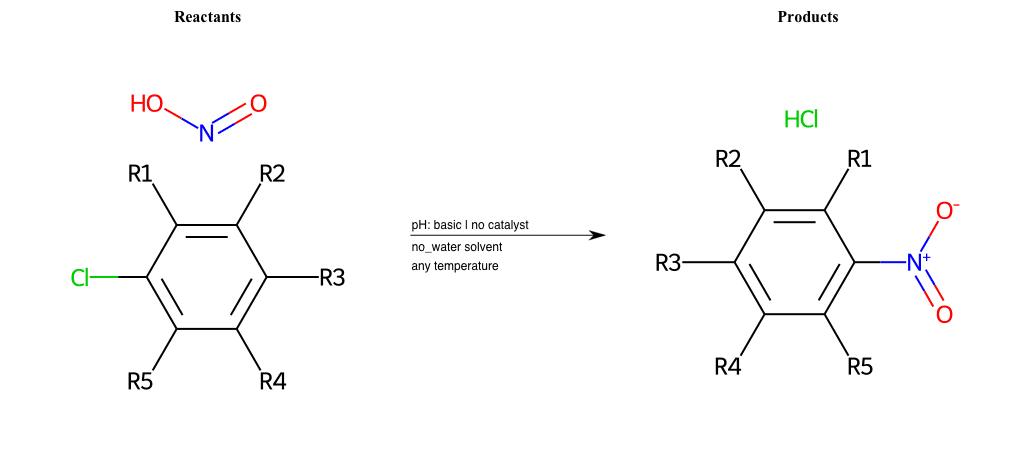
# Nucleophilic-Aromatic-Substitution-Pyridine-Para-Lg-Fluorine-and-Nu-Amino
References:
[0]
organic chemistry - Why does nucleophilic aromatic substitution occur at C-2 and C-4 of pyridine? - Chemistry Stack Exchange
[1]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[2]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[3]
Nucleophilic Aromatic Substitution - Chemistry Steps
[4]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
Condition to enforce:
R5 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
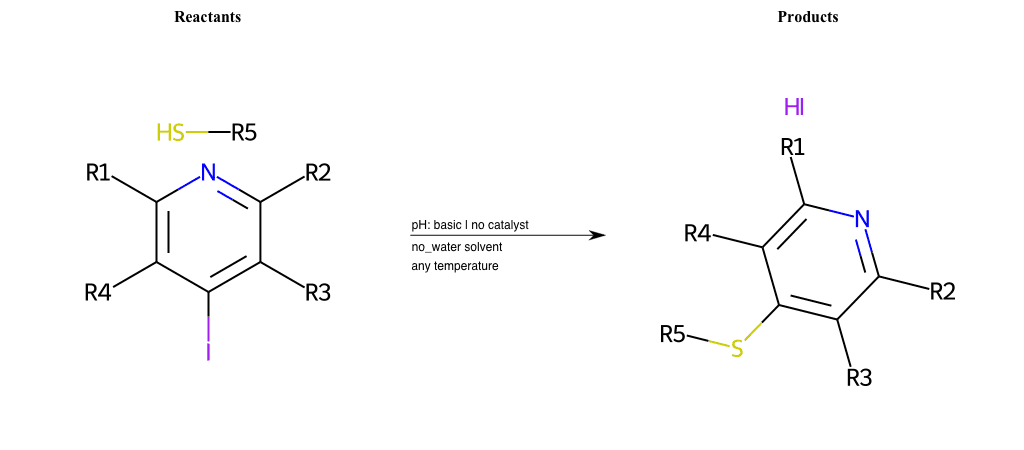
# Nucleophilic-Aromatic-Substitution-Lg-Chlorine-and-Nu-Thiolate
References:
[0]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[1]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[2]
Nucleophilic Aromatic Substitution - Chemistry Steps
[3]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
[4]
Explain why the trifluoromethyl (CF_3) group is meta directing in electrophilic aromatic substitution. What would you expect CF_3 to be activating or deactivating? Why? Can some one PLEASE help me!!!!! Thanks? | Socratic
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
# Nucleophilic-Aromatic-Substitution-Lg-Chlorine-and-Nu-Hydroxyl
References:
[0]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[1]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[2]
Nucleophilic Aromatic Substitution - Chemistry Steps
[3]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
[4]
Explain why the trifluoromethyl (CF_3) group is meta directing in electrophilic aromatic substitution. What would you expect CF_3 to be activating or deactivating? Why? Can some one PLEASE help me!!!!! Thanks? | Socratic
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
# Vicarious-Nucleophilic-Substitution-Para-X-Sulfonate-and-EWG-Carbonyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R7 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
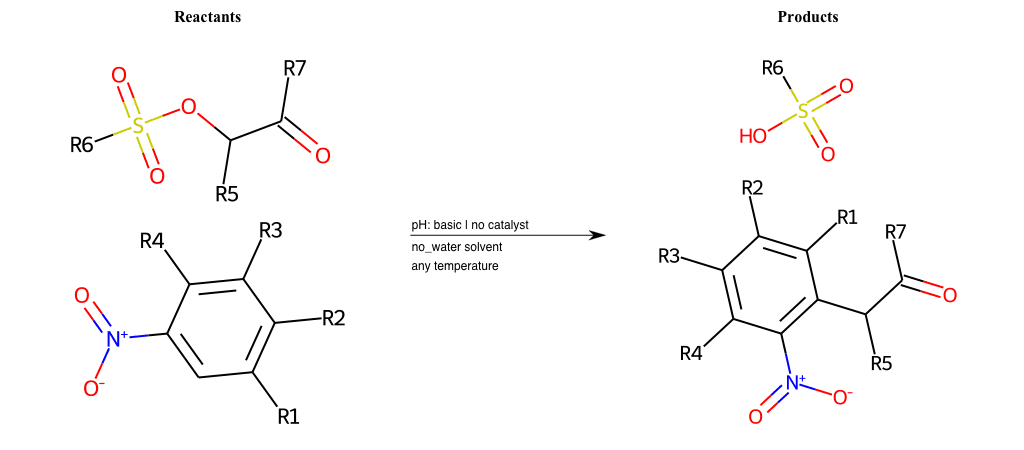
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carbonyl-and-EWG2-Carboxyl-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
R8 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
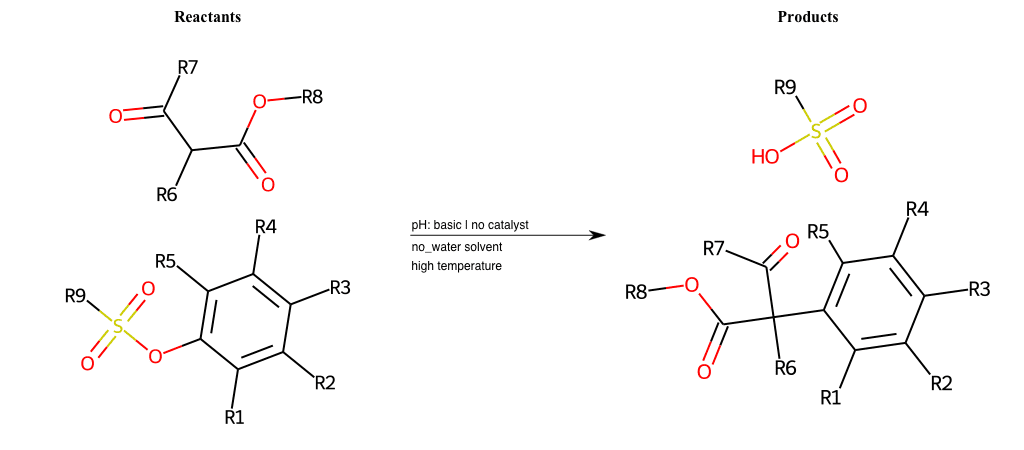
# Vicarious-Nucleophilic-Substitution-Ortho-X-Thiolate-and-EWG-Carbonyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R7 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
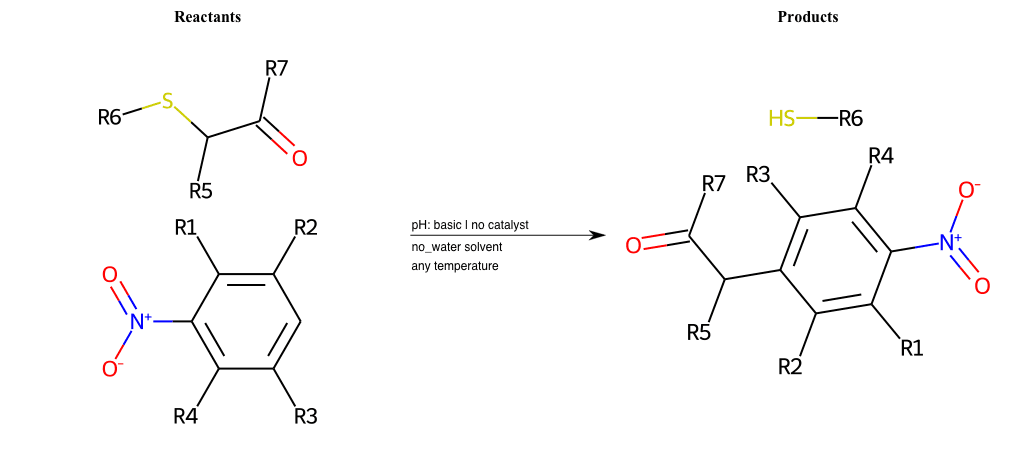
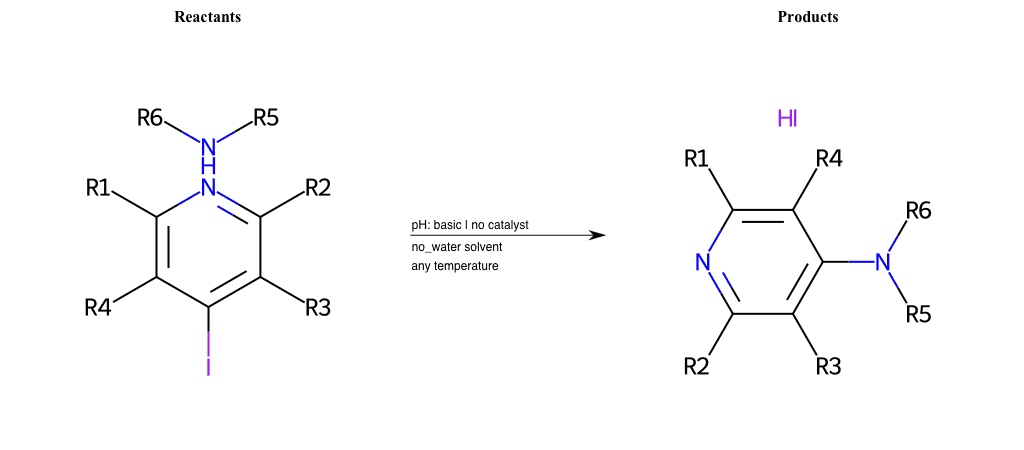
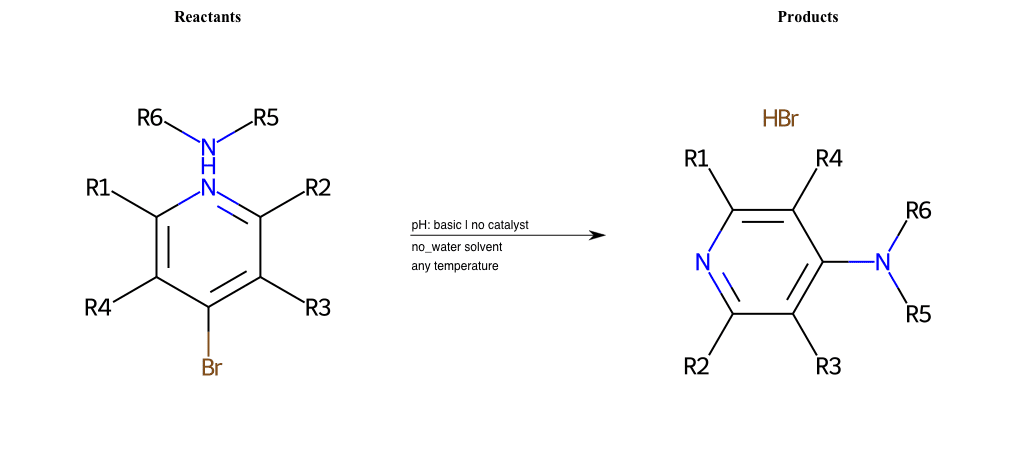
# Substituted-Pyridine-Nucleophilic-Aromatic-Substitution-Lg-Bromine-and-Nu-Amino
References:
[0]
Weickgenannt_Jun_12.pdf
Condition to enforce:
R4 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
R5 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
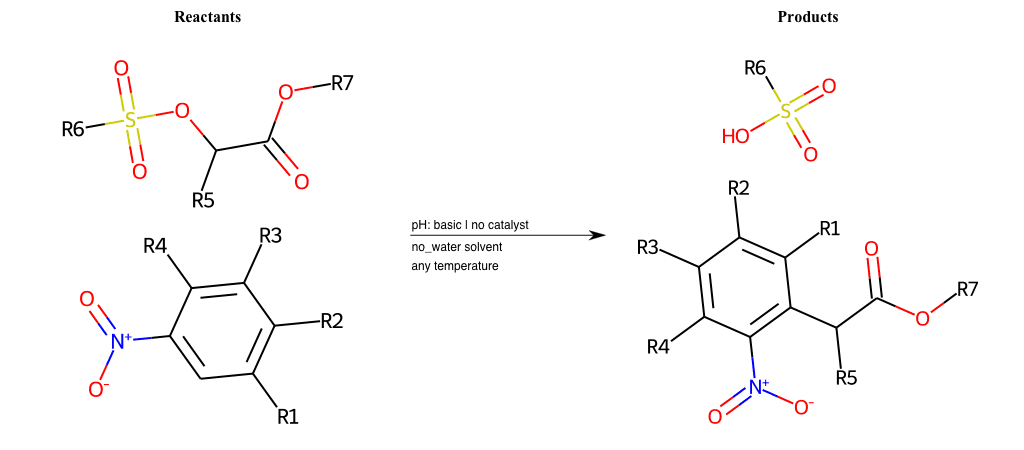
# Vicarious-Nucleophilic-Substitution-Para-X-Sulfonate-and-EWG-Carboxyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R7 = A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
# Vicarious-Nucleophilic-Substitution-Ortho-X-Thiolate-and-EWG-Carboxyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R7 = A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
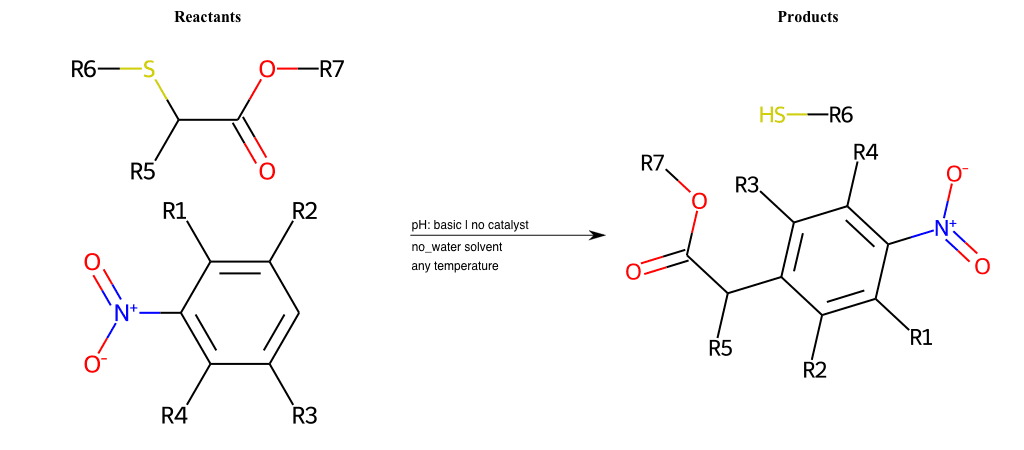
# Vicarious-Nucleophilic-Substitution-Ortho-X-Bromine-and-EWG-Sulfonyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
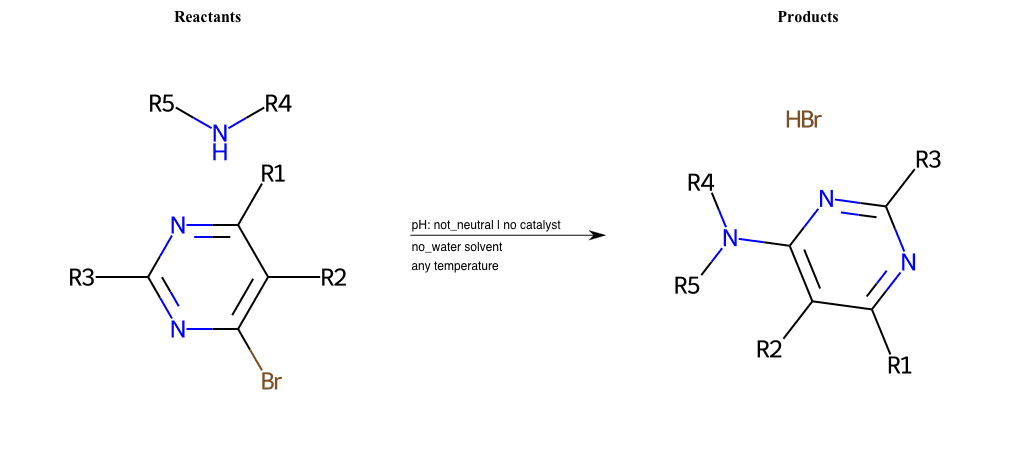
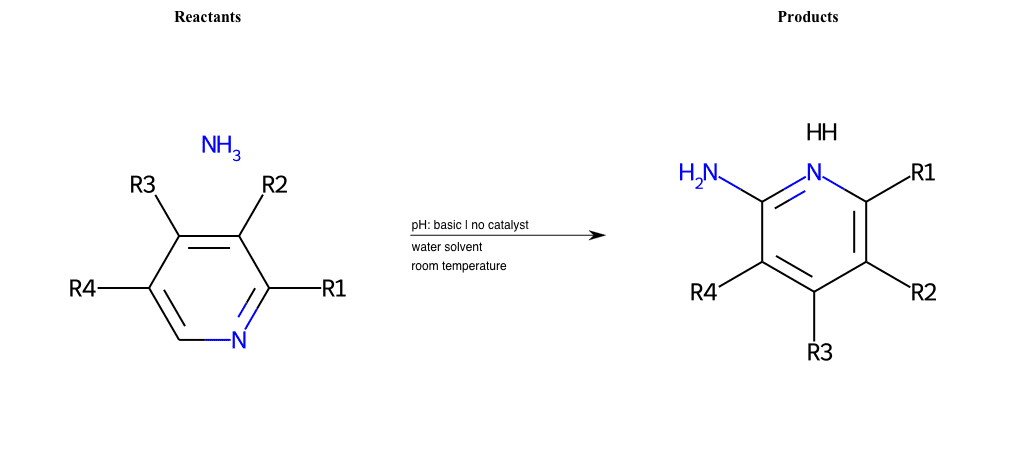
# Chihibabin-Reaction
References:
[0]
Chichibabin reaction - Wikipedia
Condition to enforce:
R1 = L-A, A-Aromatic-Carbon
R2 = L-A, A-Aromatic-Carbon
R3 = L-A, A-Aromatic-Carbon
R4 = L-A, A-Aromatic-Carbon
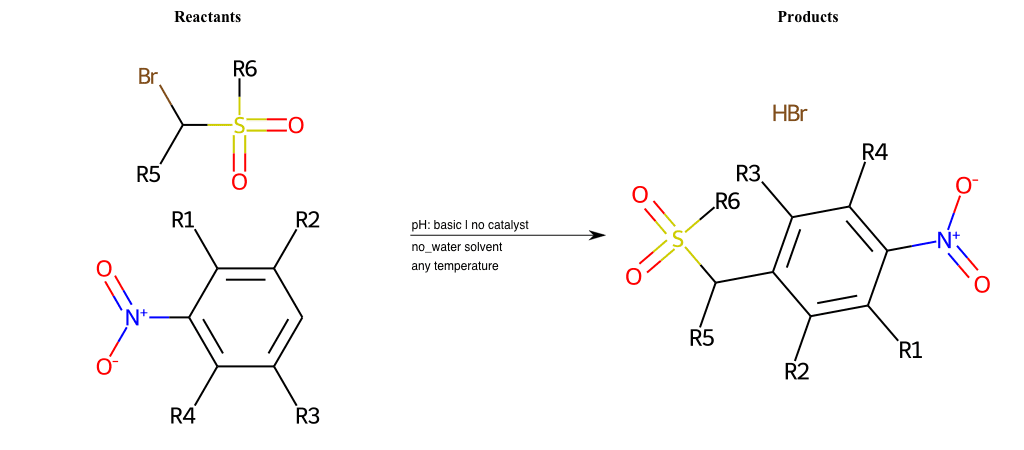
# Vicarious-Nucleophilic-Substitution-Para-X-Thiolate-and-EWG-Nitrile
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
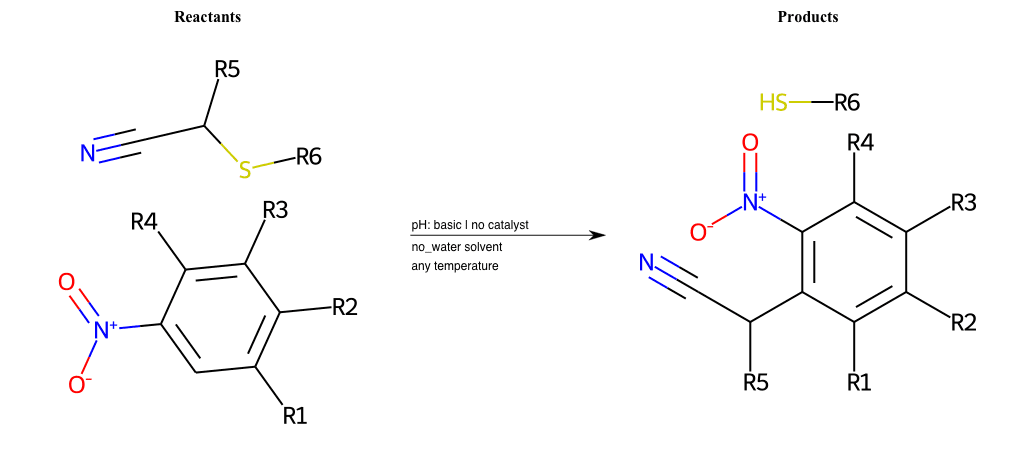
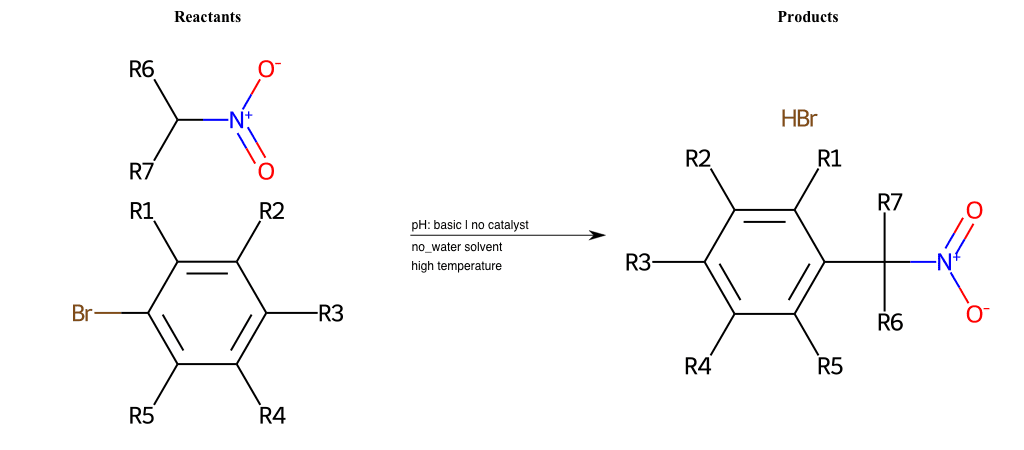
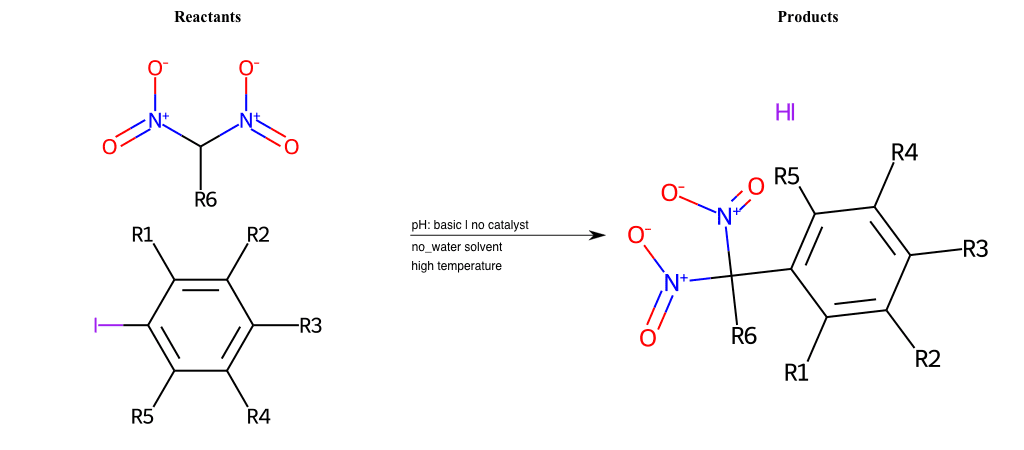
# Nucleophilic-Aromatic-Substitution-Lg-Bromine-and-Nu-Amino
References:
[0]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[1]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[2]
Nucleophilic Aromatic Substitution - Chemistry Steps
[3]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
[4]
Explain why the trifluoromethyl (CF_3) group is meta directing in electrophilic aromatic substitution. What would you expect CF_3 to be activating or deactivating? Why? Can some one PLEASE help me!!!!! Thanks? | Socratic
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
R7 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
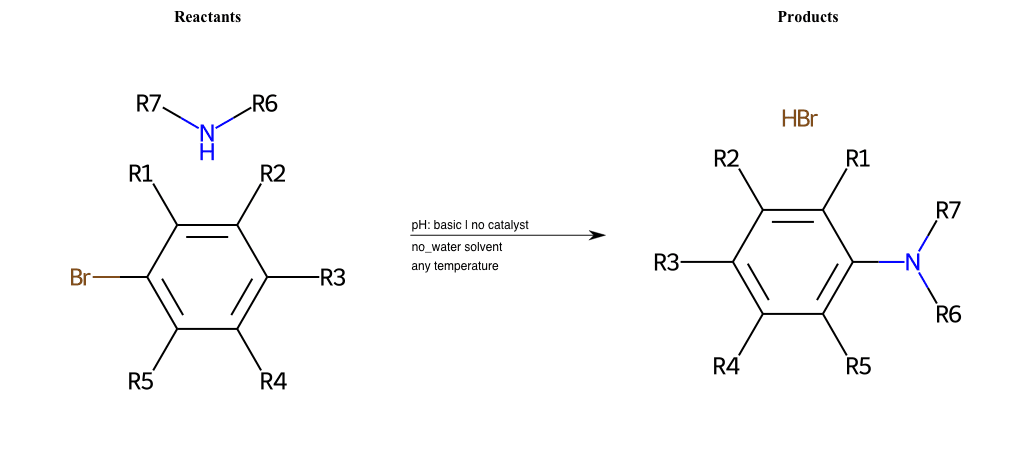
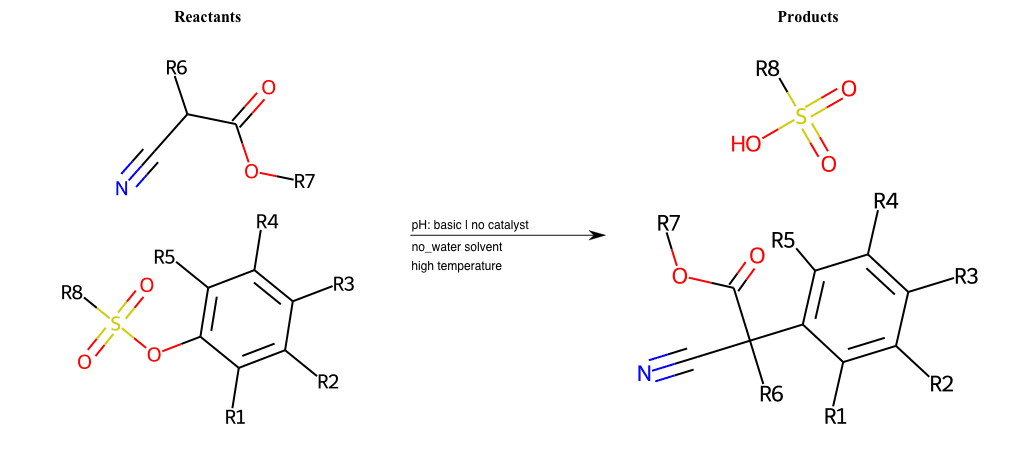
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carboxyl-and-EWG2-Nitrile-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
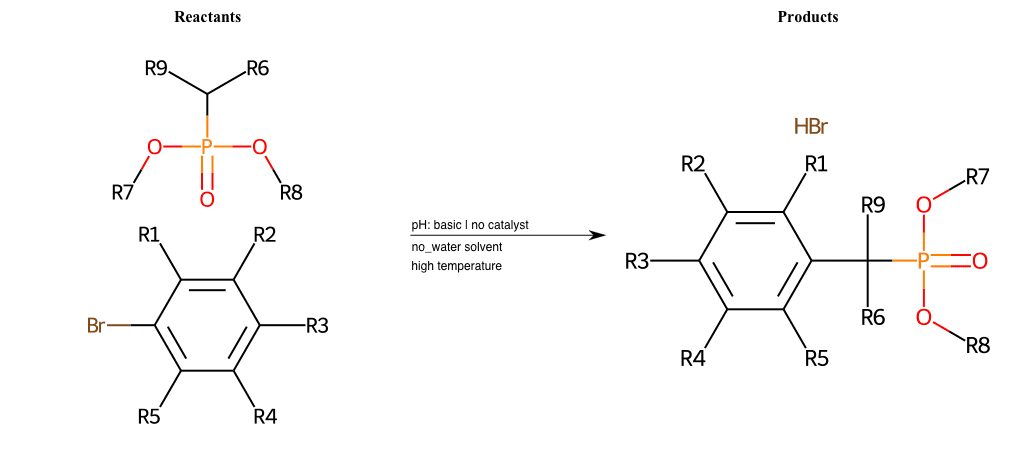
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Phosphonate-and-EWG2-Alkane-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R8 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R9 = H, A-Aliphatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
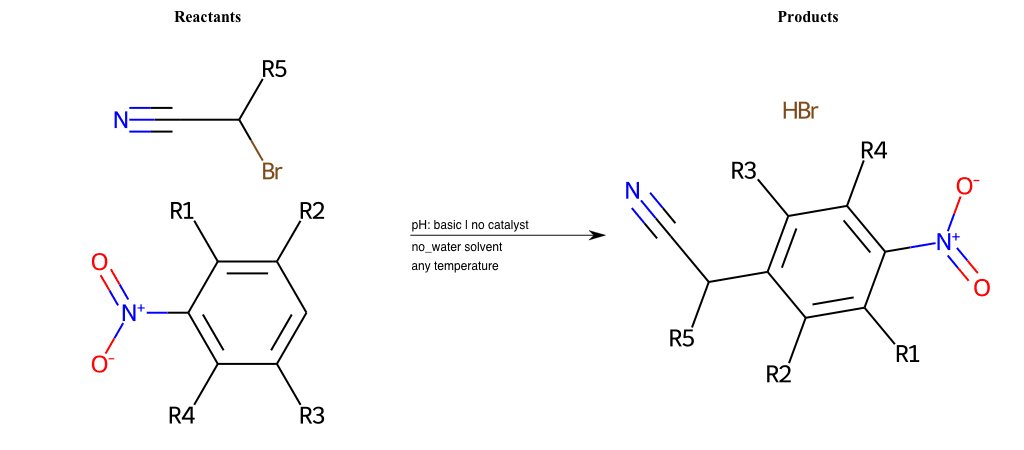
# Vicarious-Nucleophilic-Substitution-Ortho-X-Bromine-and-EWG-Nitrile
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
# Nucleophilic-Aromatic-Substitution-Pyridine-Para-Lg-Bromine-and-Nu-Alkoxide
References:
[0]
organic chemistry - Why does nucleophilic aromatic substitution occur at C-2 and C-4 of pyridine? - Chemistry Stack Exchange
[1]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[2]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[3]
Nucleophilic Aromatic Substitution - Chemistry Steps
[4]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
Condition to enforce:
R5 = A-Aliphatic-Carbon, A-Aromatic-Carbon
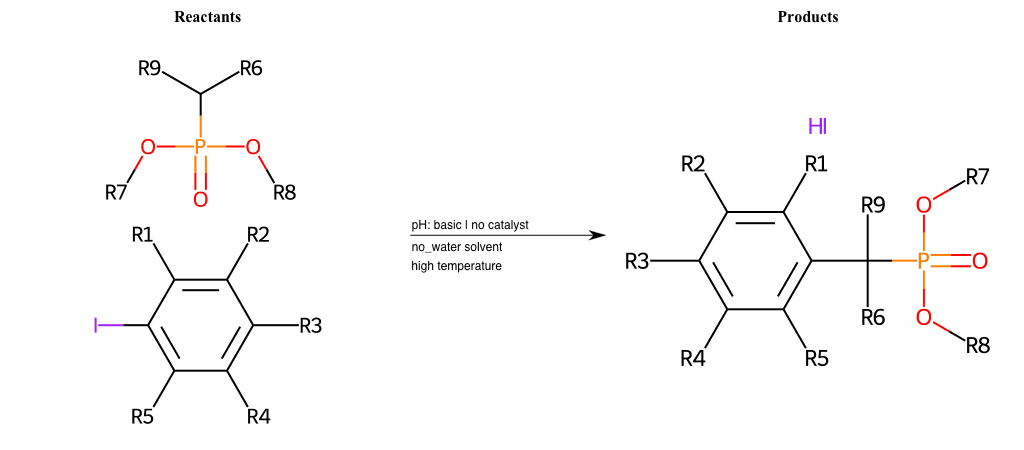
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Phosphonate-and-EWG2-Alkane-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R8 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R9 = H, A-Aliphatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
# Vicarious-Nucleophilic-Substitution-Ortho-X-Alkoxide-and-EWG-Nitrile
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
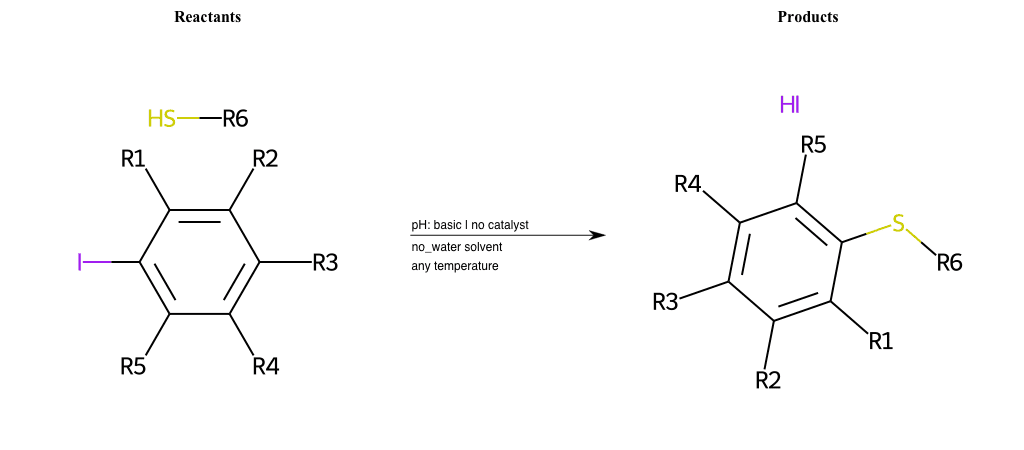
# Nucleophilic-Aromatic-Substitution-Lg-Iodine-and-Nu-Thiolate
References:
[0]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[1]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[2]
Nucleophilic Aromatic Substitution - Chemistry Steps
[3]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
[4]
Explain why the trifluoromethyl (CF_3) group is meta directing in electrophilic aromatic substitution. What would you expect CF_3 to be activating or deactivating? Why? Can some one PLEASE help me!!!!! Thanks? | Socratic
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
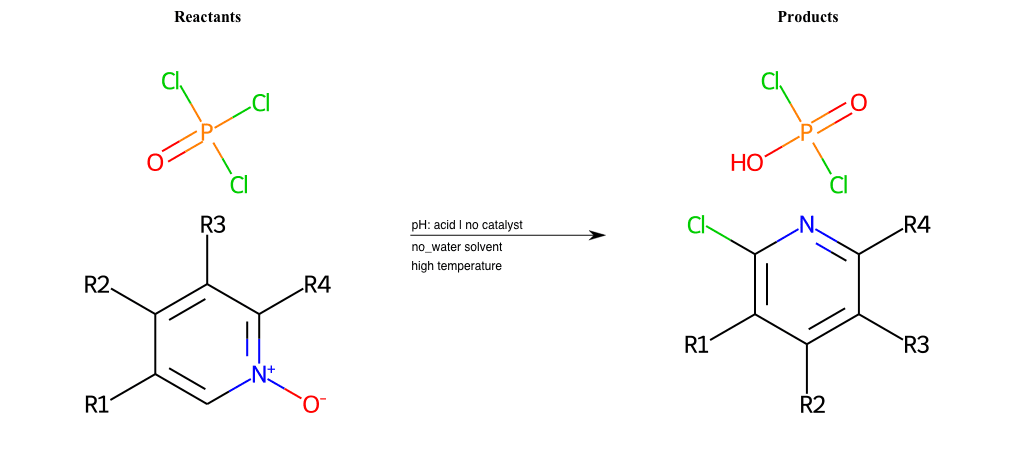
# Pyridine-N-Oxide-Chlorination-Ortho
References:
[0]
Weickgenannt_Jun_12.pdf
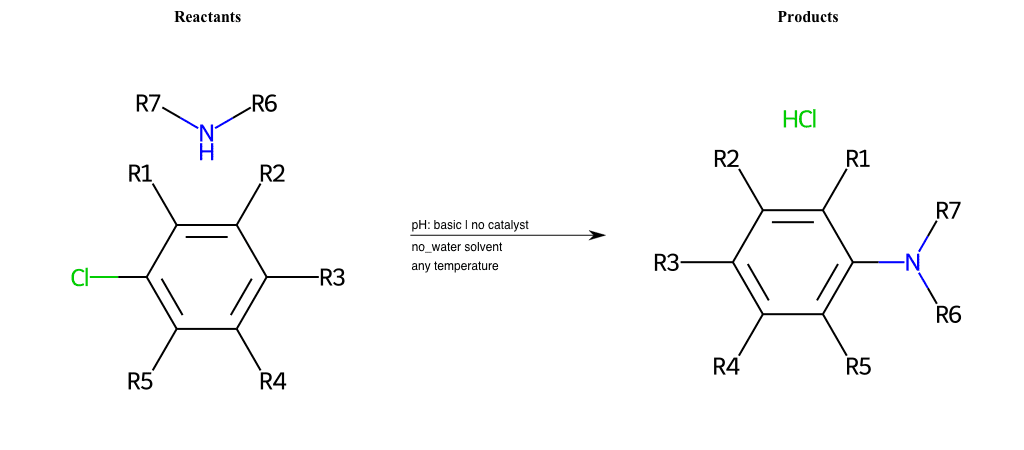
# Nucleophilic-Aromatic-Substitution-Lg-Chlorine-and-Nu-Amino
References:
[0]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[1]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[2]
Nucleophilic Aromatic Substitution - Chemistry Steps
[3]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
[4]
Explain why the trifluoromethyl (CF_3) group is meta directing in electrophilic aromatic substitution. What would you expect CF_3 to be activating or deactivating? Why? Can some one PLEASE help me!!!!! Thanks? | Socratic
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
R7 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
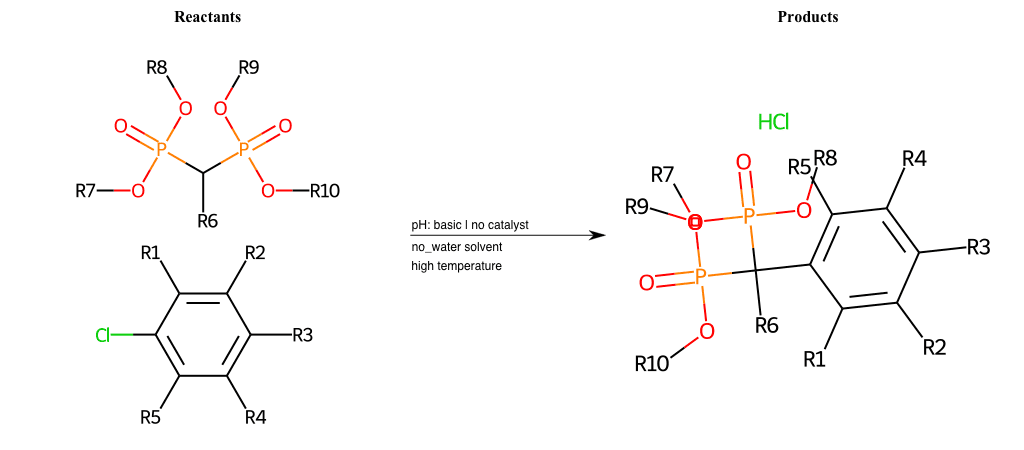
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Phosphonate-and-EWG2-Phosphonate-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R8 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R9 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R10 = A-Aliphatic-Carbon, A-Aromatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
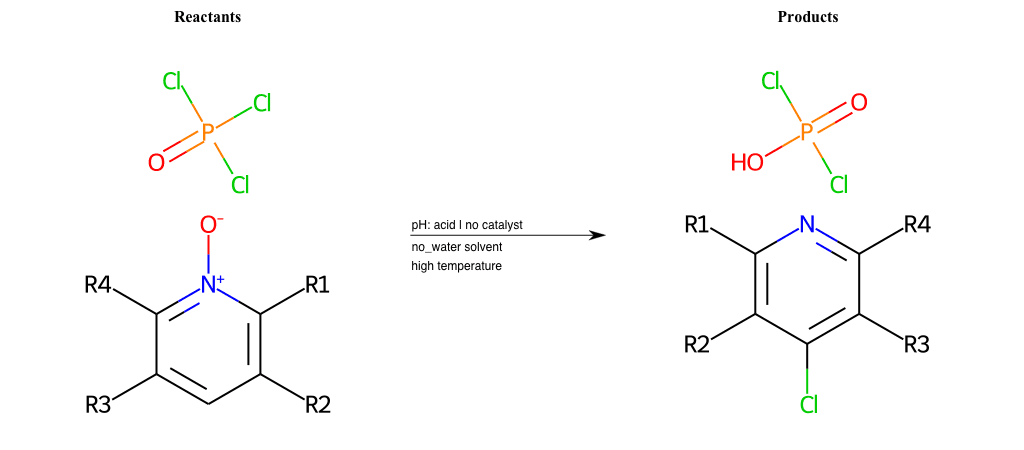
# Pyridine-N-Oxide-Chlorination-Para
References:
[0]
Weickgenannt_Jun_12.pdf
# Vicarious-Nucleophilic-Substitution-Ortho-X-Iodine-and-EWG-Nitrile
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
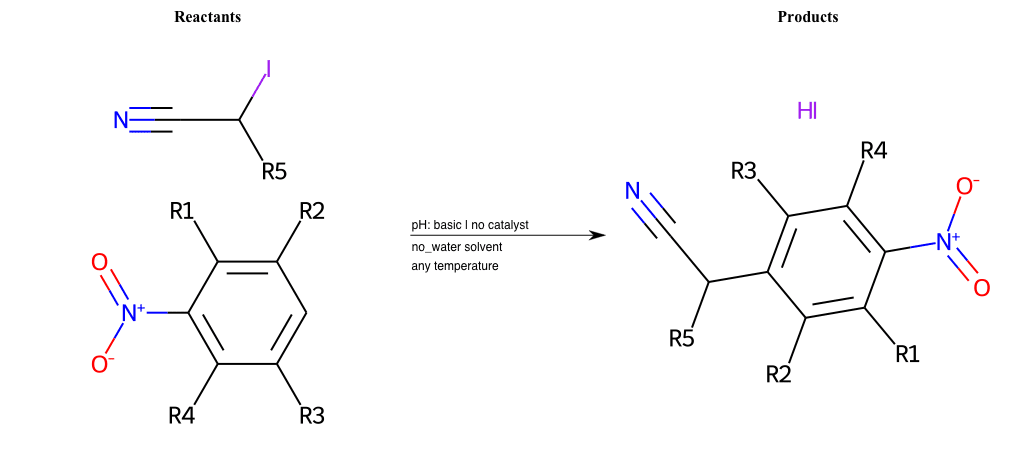
# Vicarious-Nucleophilic-Substitution-Para-X-Iodine-and-EWG-Nitrile
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
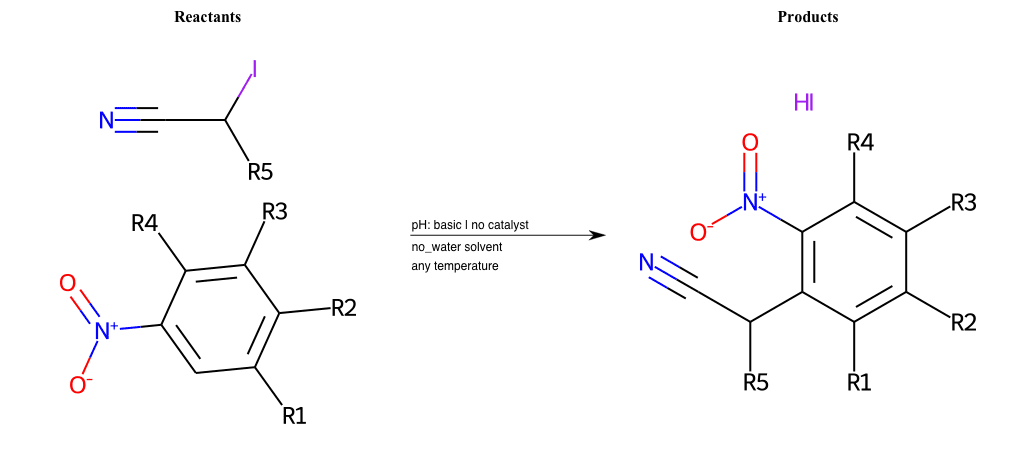
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carboxyl-and-EWG2-Nitrite-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
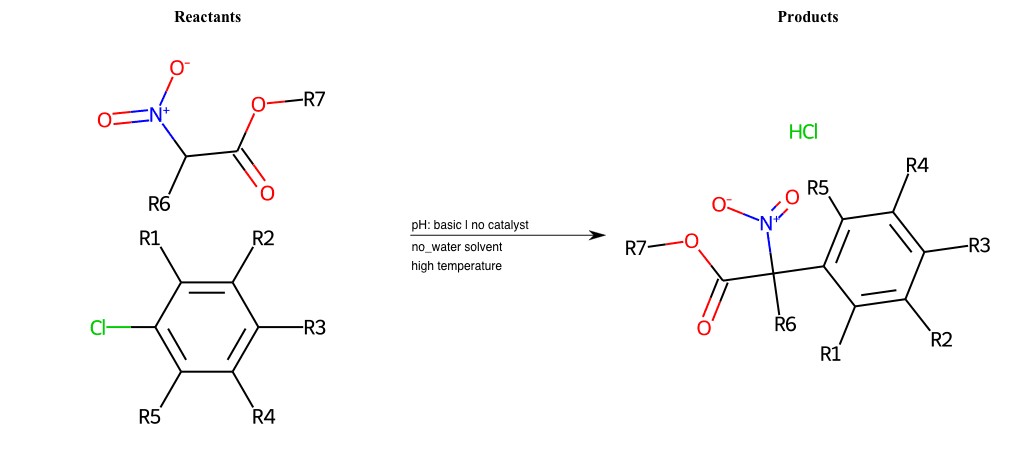
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carbonyl-and-EWG2-Alkane-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
R8 = H, A-Aliphatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
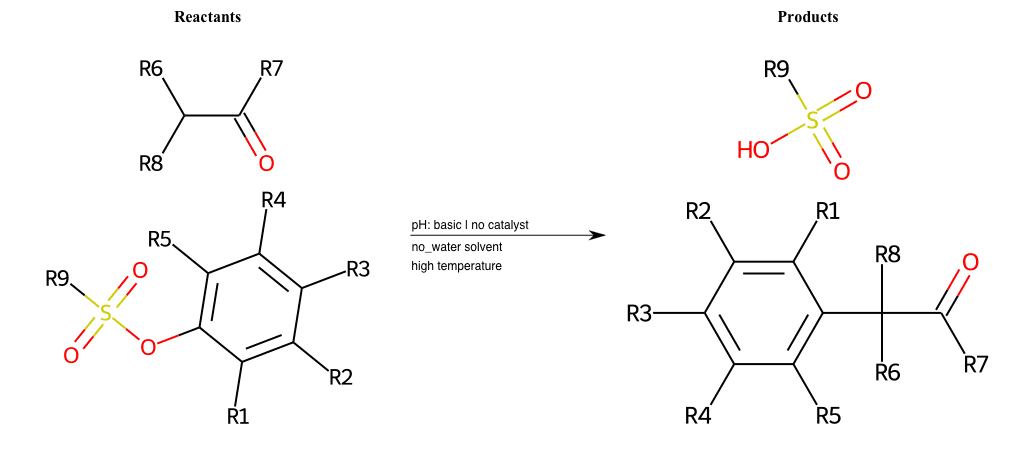
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carbonyl-and-EWG2-Alkane-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
R8 = H, A-Aliphatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
# Nucleophilic-Aromatic-Substitution-Pyridine-Para-Lg-Iodine-and-Nu-Amino
References:
[0]
organic chemistry - Why does nucleophilic aromatic substitution occur at C-2 and C-4 of pyridine? - Chemistry Stack Exchange
[1]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[2]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[3]
Nucleophilic Aromatic Substitution - Chemistry Steps
[4]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
Condition to enforce:
R5 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
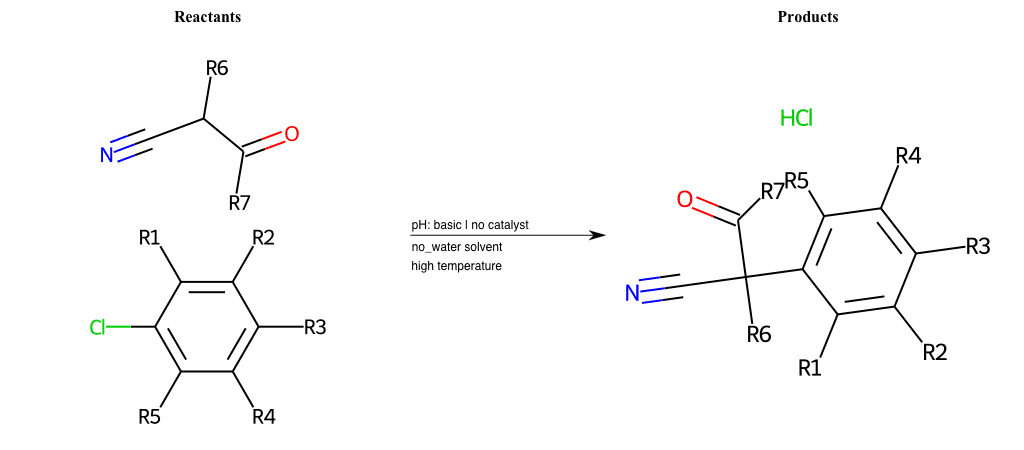
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carbonyl-and-EWG2-Nitrile-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carbonyl-and-EWG2-Nitrite-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
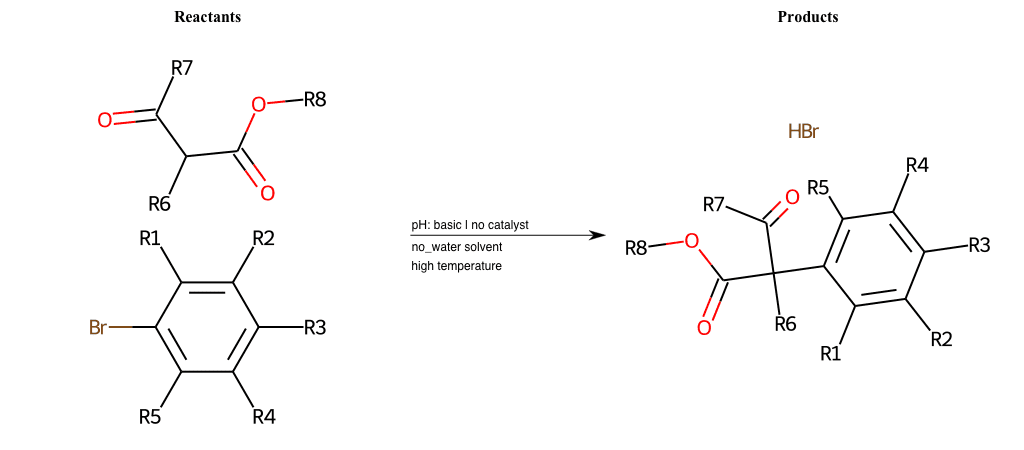
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carboxyl-and-EWG2-Carboxyl-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
R8 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
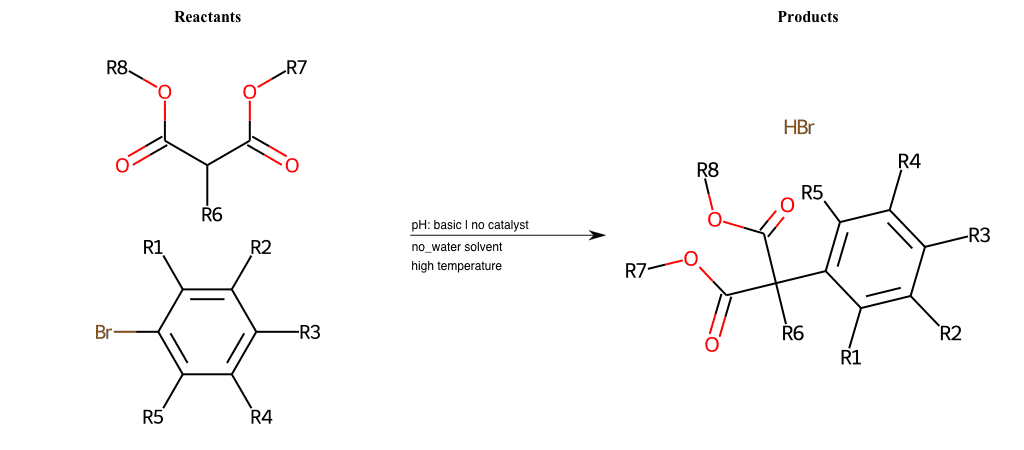
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Nitrite-and-EWG2-Nitrite-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
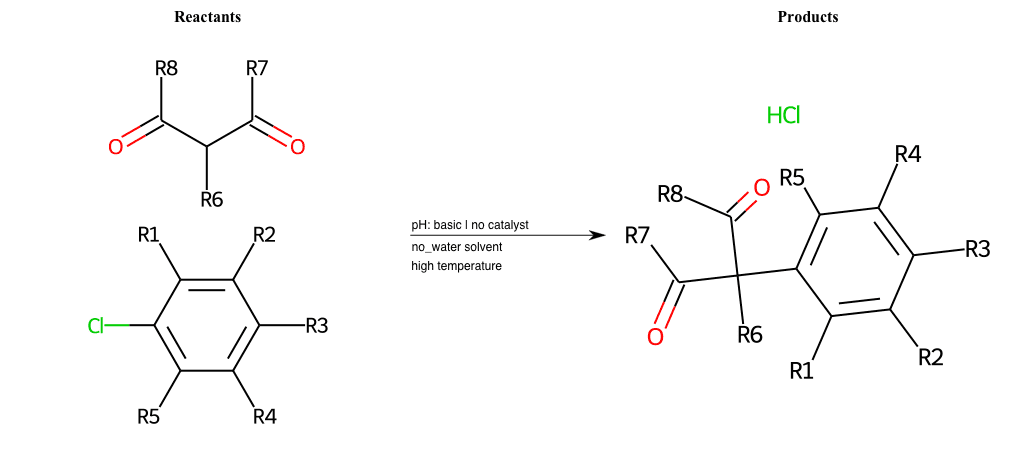
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carbonyl-and-EWG2-Carbonyl-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
R8 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
# Vicarious-Nucleophilic-Substitution-Para-X-Thiolate-and-EWG-Sulfonyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R7 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
# Nucleophilic-Aromatic-Substitution-Pyridine-Ortho-Lg-Bromine-and-Nu-Alkoxide
References:
[0]
organic chemistry - Why does nucleophilic aromatic substitution occur at C-2 and C-4 of pyridine? - Chemistry Stack Exchange
[1]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[2]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[3]
Nucleophilic Aromatic Substitution - Chemistry Steps
[4]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
Condition to enforce:
R5 = A-Aliphatic-Carbon, A-Aromatic-Carbon
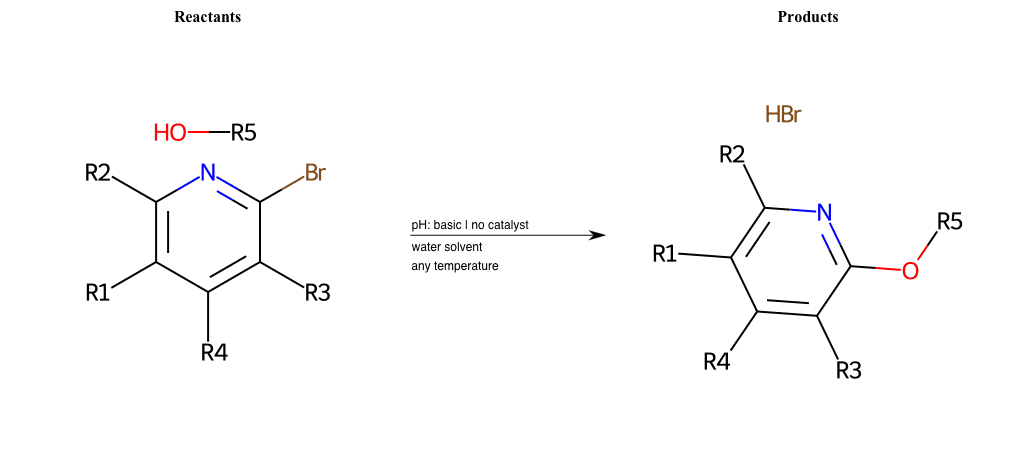
# Nucleophilic-Aromatic-Substitution-Pyridine-Para-Lg-Bromine-and-Nu-Amino
References:
[0]
organic chemistry - Why does nucleophilic aromatic substitution occur at C-2 and C-4 of pyridine? - Chemistry Stack Exchange
[1]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[2]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[3]
Nucleophilic Aromatic Substitution - Chemistry Steps
[4]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
Condition to enforce:
R5 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
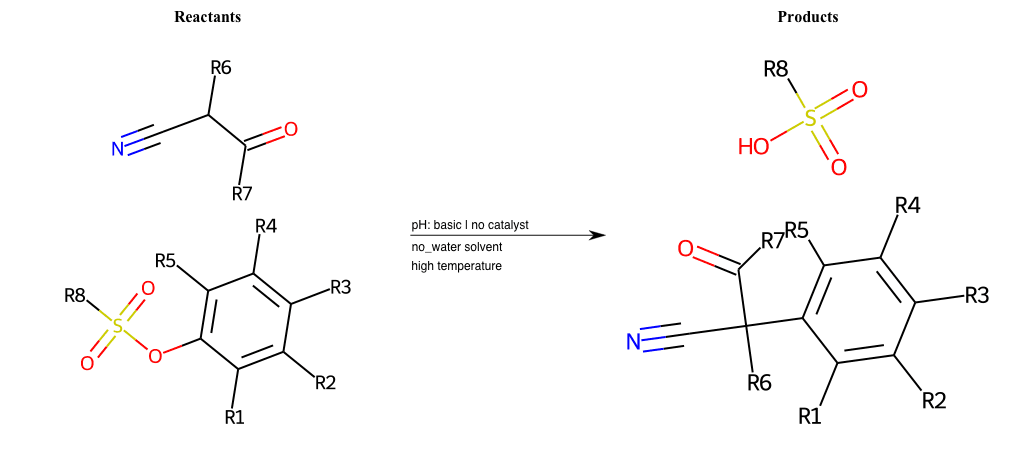
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carbonyl-and-EWG2-Nitrile-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
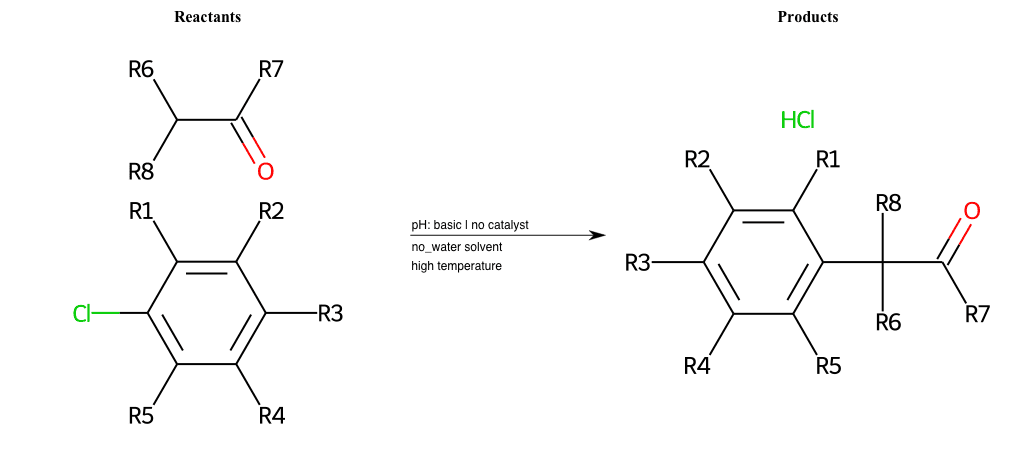
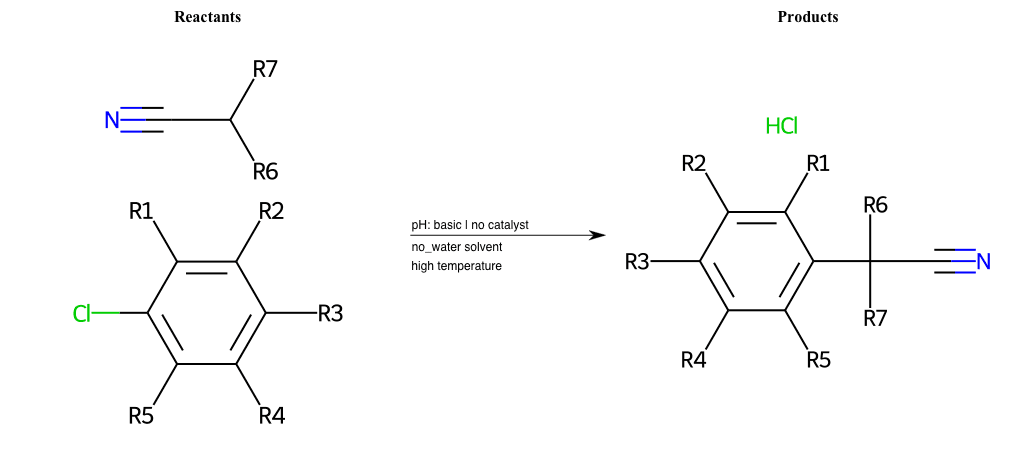
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Nitrile-and-EWG2-Alkane-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = H, A-Aliphatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carbonyl-and-EWG2-Nitrite-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
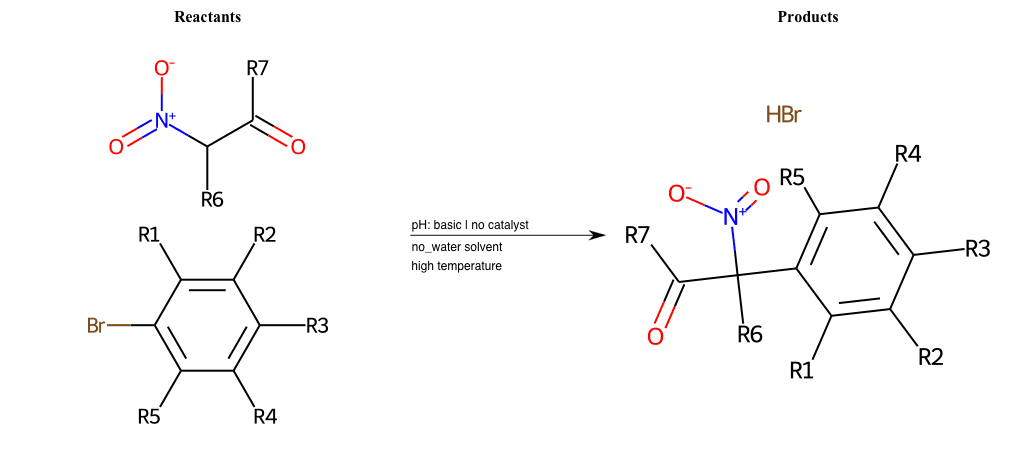
# Vicarious-Nucleophilic-Substitution-Para-X-Alkoxide-and-EWG-Nitrile
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
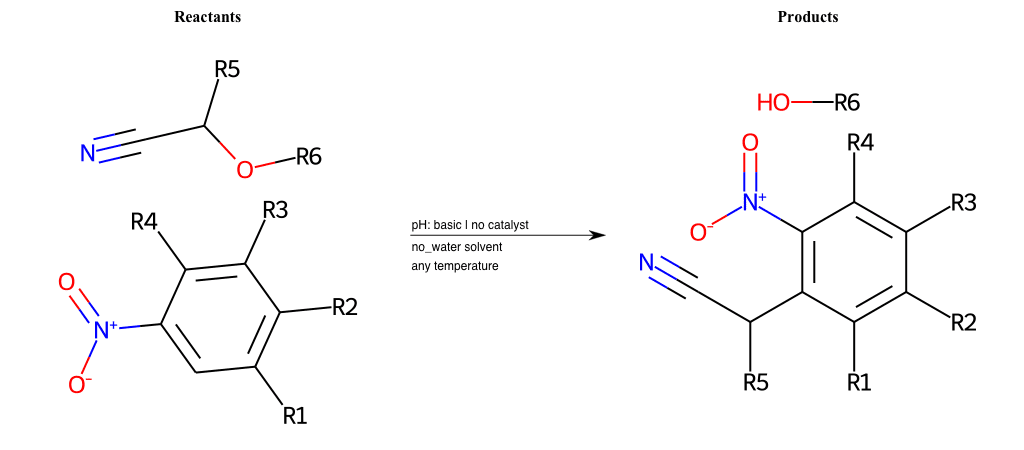
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carboxyl-and-EWG2-Carboxyl-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
R8 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
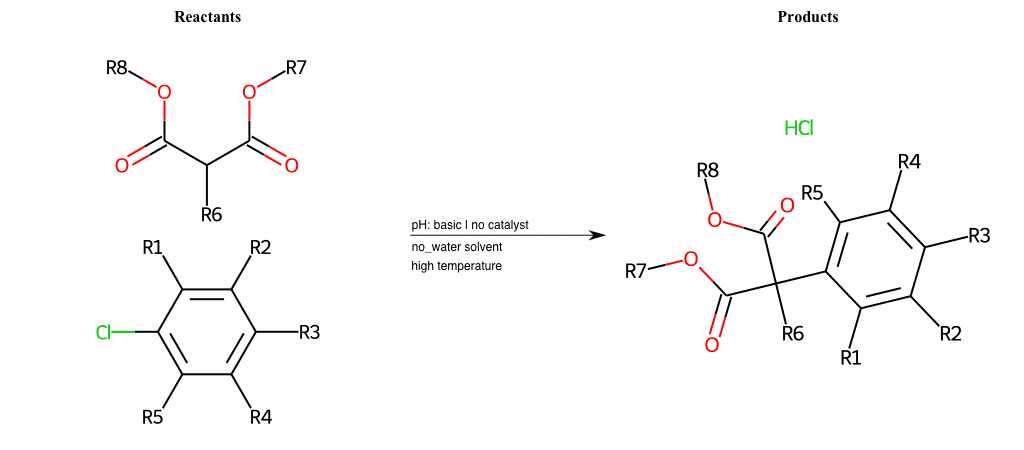
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Nitrite-and-EWG2-Phosphonate-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R8 = A-Aliphatic-Carbon, A-Aromatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
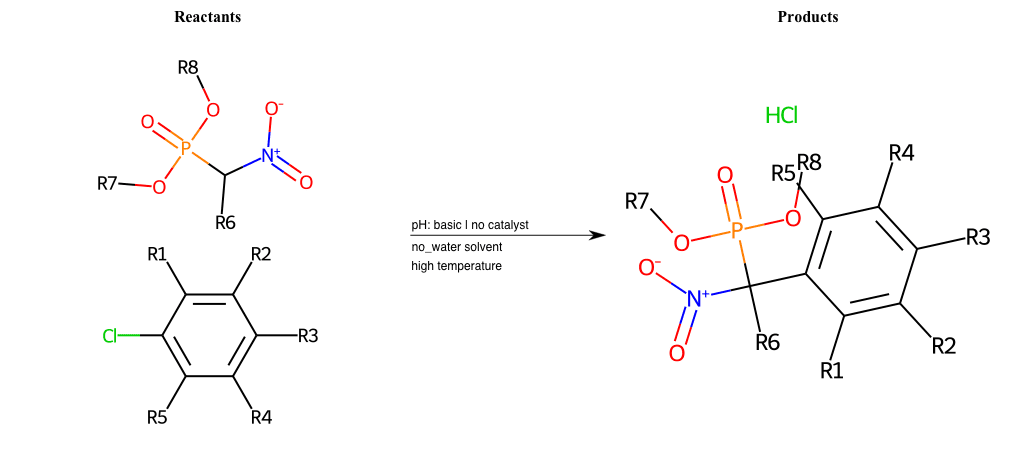
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carboxyl-and-EWG2-Carboxyl-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
R8 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
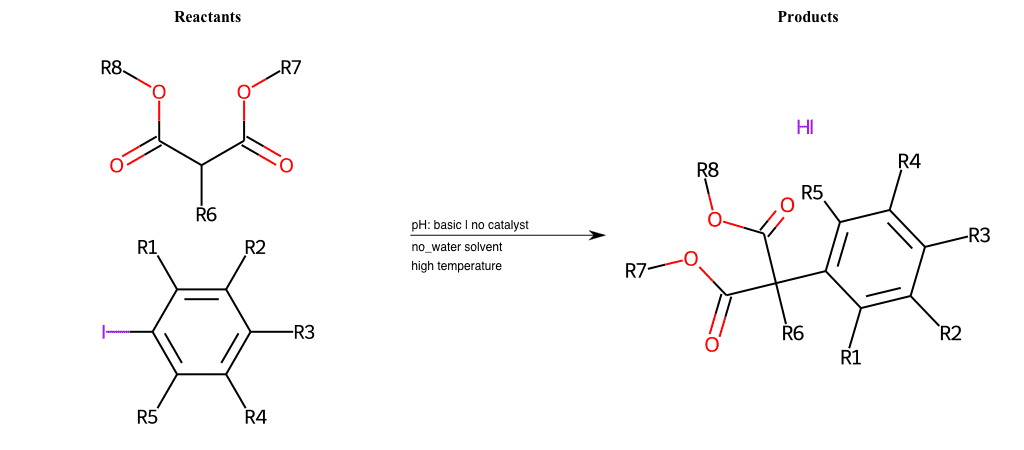
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Nitrile-and-EWG2-Nitrite-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
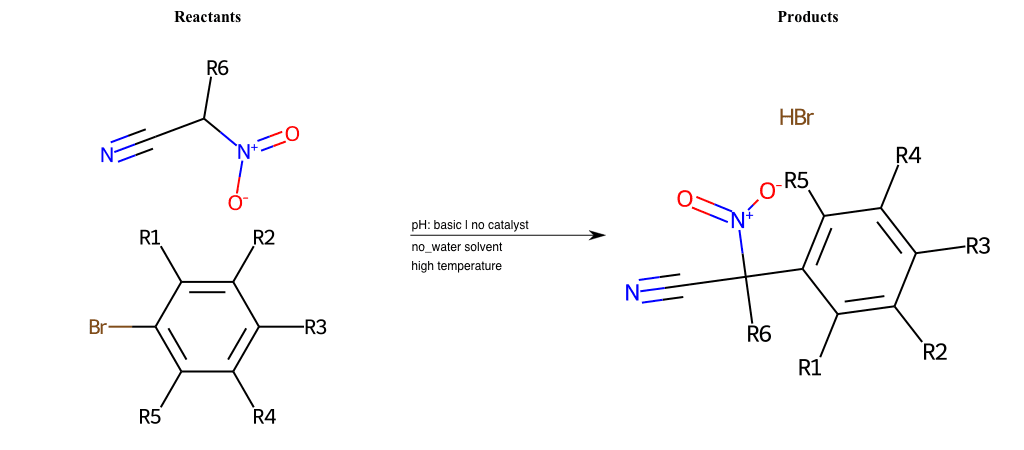
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carbonyl-and-EWG2-Phosphonate-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
R8 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R9 = A-Aliphatic-Carbon, A-Aromatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
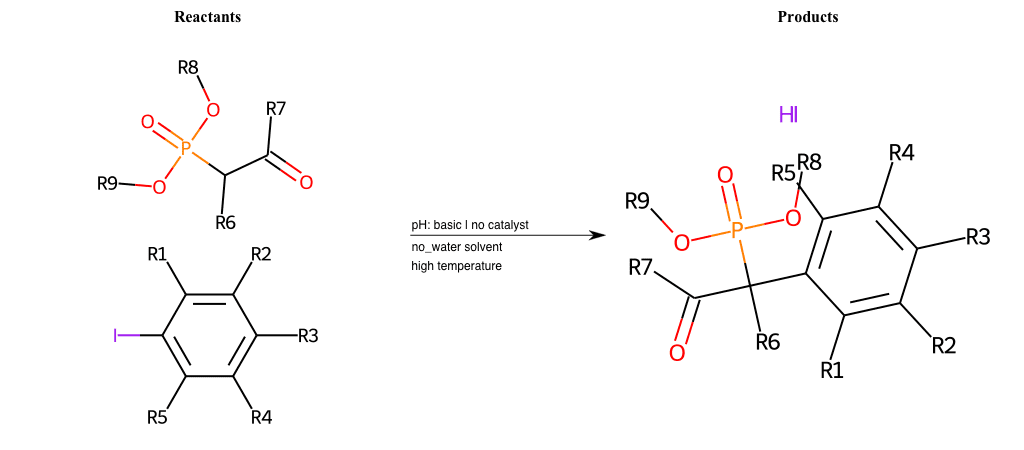
# Vicarious-Nucleophilic-Substitution-Para-X-Bromine-and-EWG-Nitrile
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
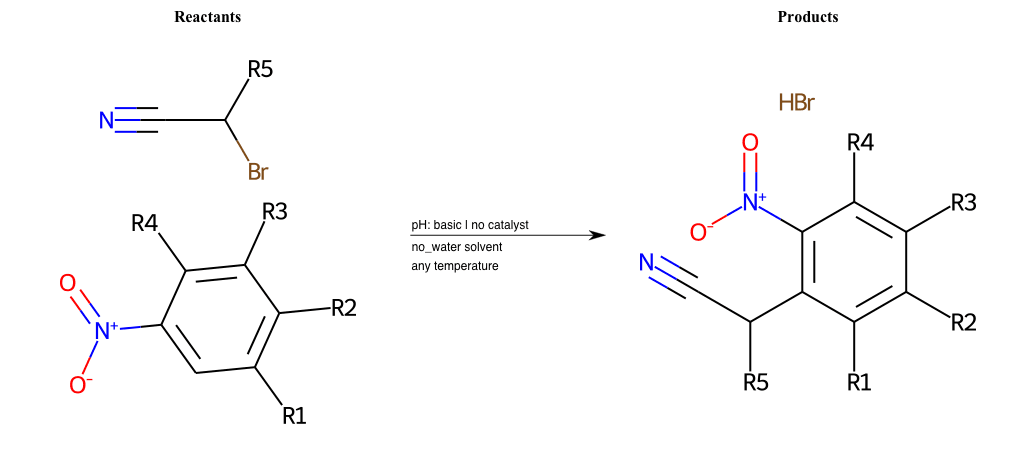
# Nucleophilic-Aromatic-Substitution-Pyridine-Para-Lg-Fluorine-and-Nu-Hydroxyl
References:
[0]
organic chemistry - Why does nucleophilic aromatic substitution occur at C-2 and C-4 of pyridine? - Chemistry Stack Exchange
[1]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[2]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[3]
Nucleophilic Aromatic Substitution - Chemistry Steps
[4]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
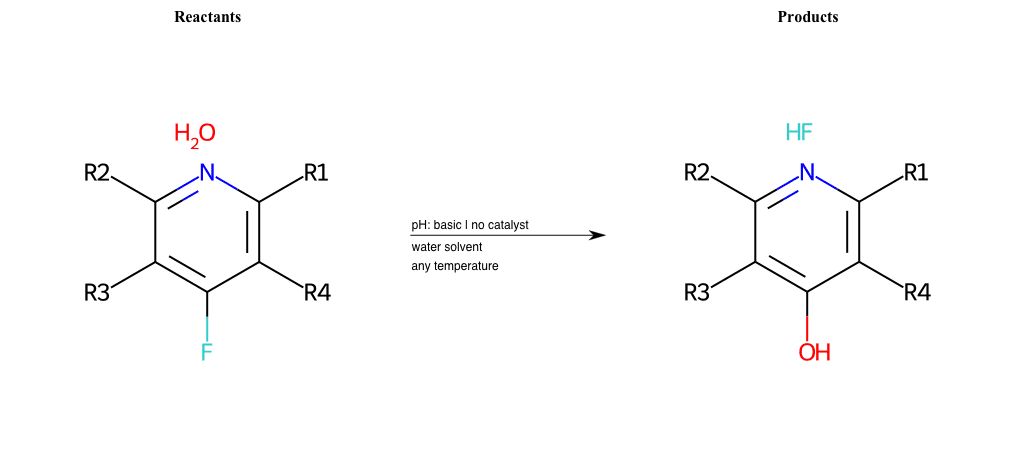
# Vicarious-Nucleophilic-Substitution-Ortho-X-Chlorine-and-EWG-Carboxyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
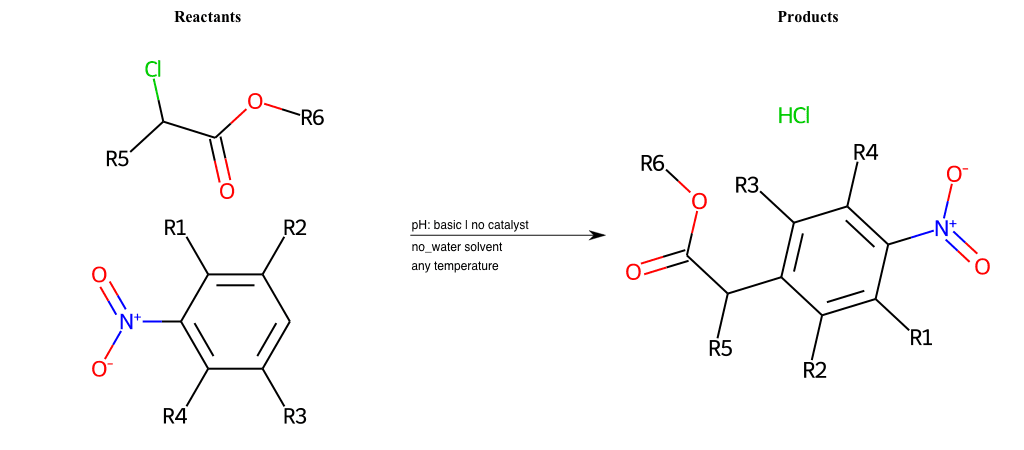
# Nucleophilic-Aromatic-Substitution-Lg-Fluorine-and-Nu-Alkoxide
References:
[0]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[1]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[2]
Nucleophilic Aromatic Substitution - Chemistry Steps
[3]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
[4]
Explain why the trifluoromethyl (CF_3) group is meta directing in electrophilic aromatic substitution. What would you expect CF_3 to be activating or deactivating? Why? Can some one PLEASE help me!!!!! Thanks? | Socratic
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = A-Aliphatic-Carbon, A-Aromatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
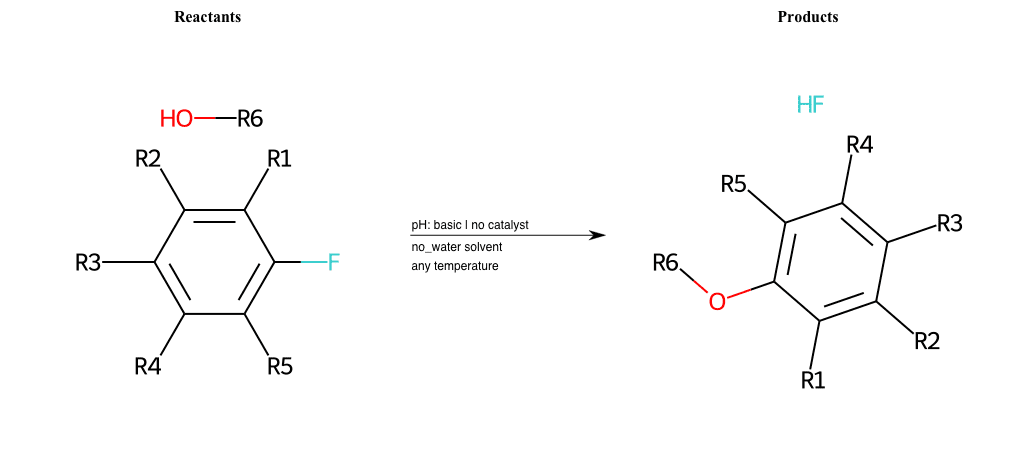
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Nitrite-and-EWG2-Nitrite-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
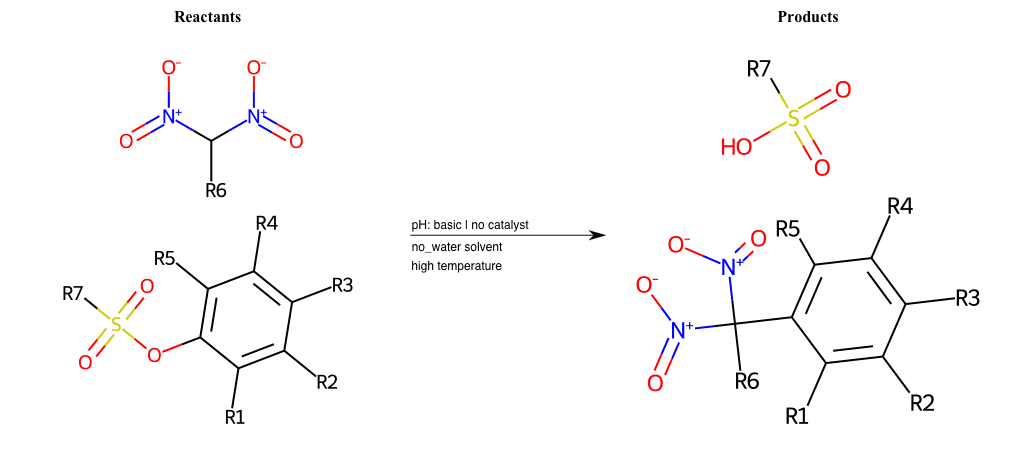
# Substituted-Pyridine-Nucleophilic-Aromatic-Substitution-Lg-Fluorine-and-Nu-Alkoxide
References:
[0]
Weickgenannt_Jun_12.pdf
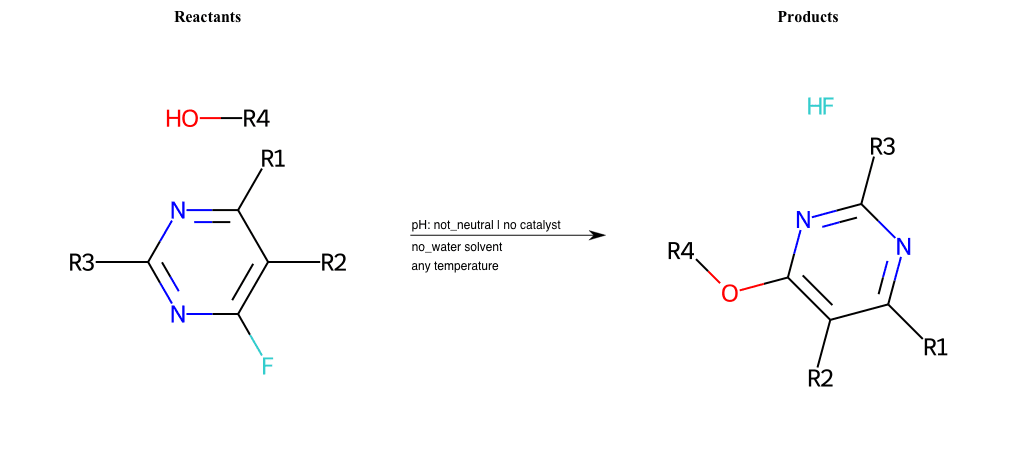
# Vicarious-Nucleophilic-Substitution-Para-X-Sulfonate-and-EWG-Nitrile
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
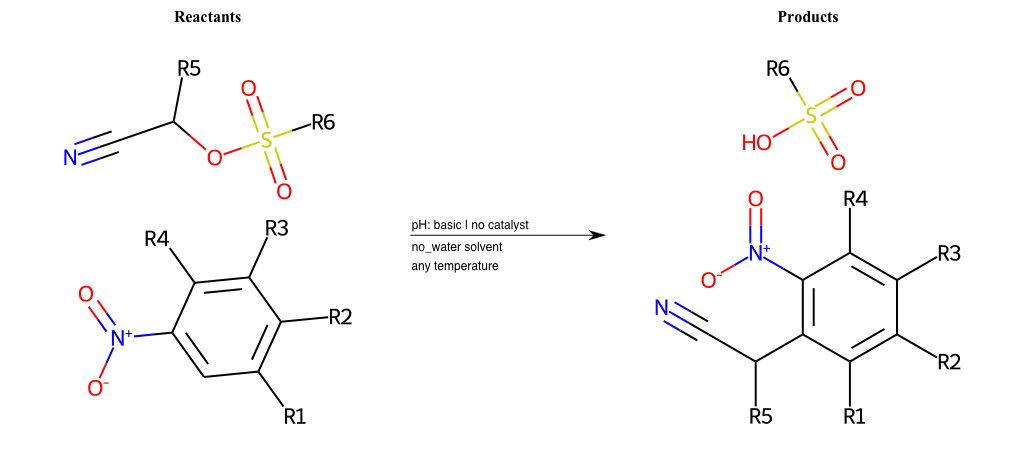
# Nucleophilic-Aromatic-Substitution-Pyridine-Ortho-Lg-Iodine-and-Nu-Alkoxide
References:
[0]
organic chemistry - Why does nucleophilic aromatic substitution occur at C-2 and C-4 of pyridine? - Chemistry Stack Exchange
[1]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[2]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[3]
Nucleophilic Aromatic Substitution - Chemistry Steps
[4]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
Condition to enforce:
R5 = A-Aliphatic-Carbon, A-Aromatic-Carbon
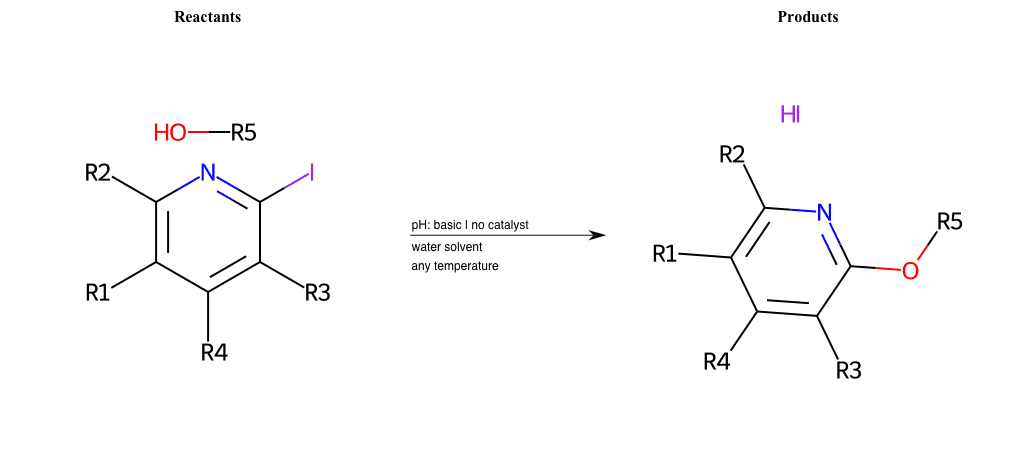
# Nucleophilic-Aromatic-Substitution-Nitric-Acid-Lg-Fluorine
References:
[0]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[1]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[2]
Nucleophilic Aromatic Substitution - Chemistry Steps
[3]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
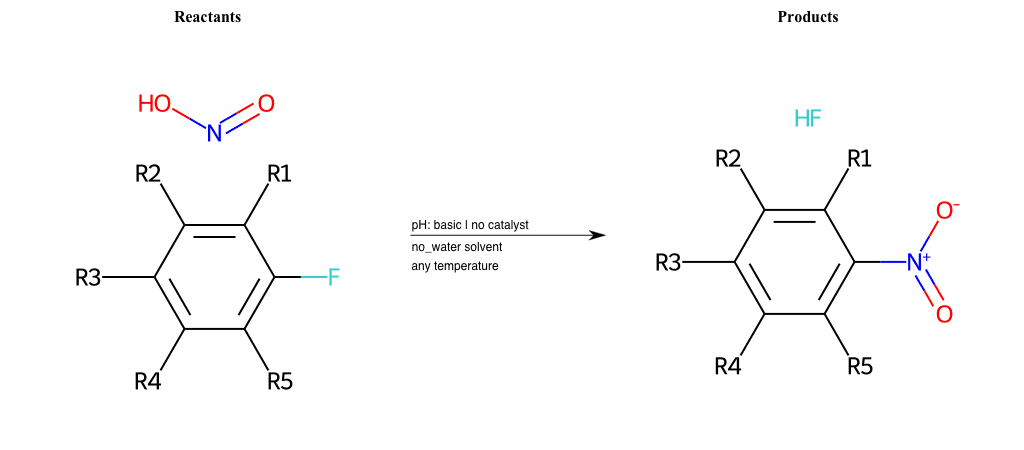
# Nucleophilic-Aromatic-Substitution-Pyridine-Para-Lg-Chlorine-and-Nu-Alkoxide
References:
[0]
organic chemistry - Why does nucleophilic aromatic substitution occur at C-2 and C-4 of pyridine? - Chemistry Stack Exchange
[1]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[2]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[3]
Nucleophilic Aromatic Substitution - Chemistry Steps
[4]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
Condition to enforce:
R5 = A-Aliphatic-Carbon, A-Aromatic-Carbon
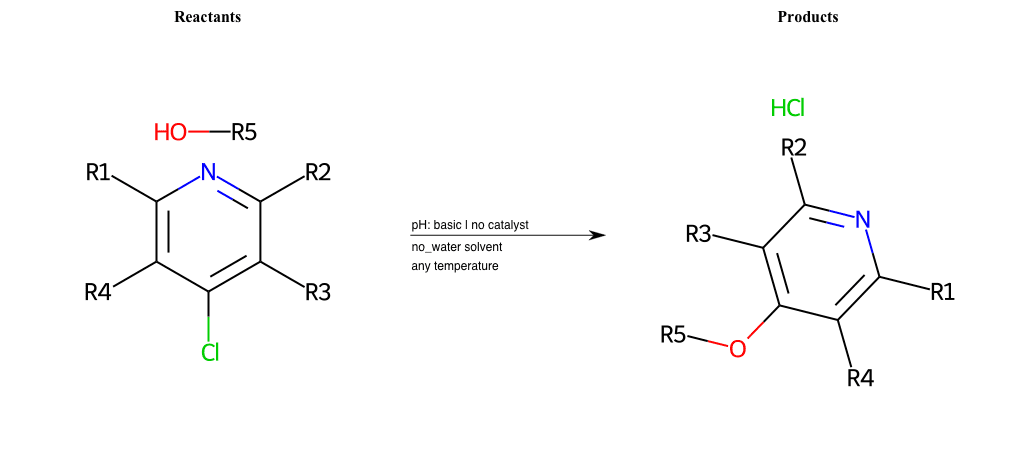
# Vicarious-Nucleophilic-Substitution-Para-X-Bromine-and-EWG-Sulfonyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
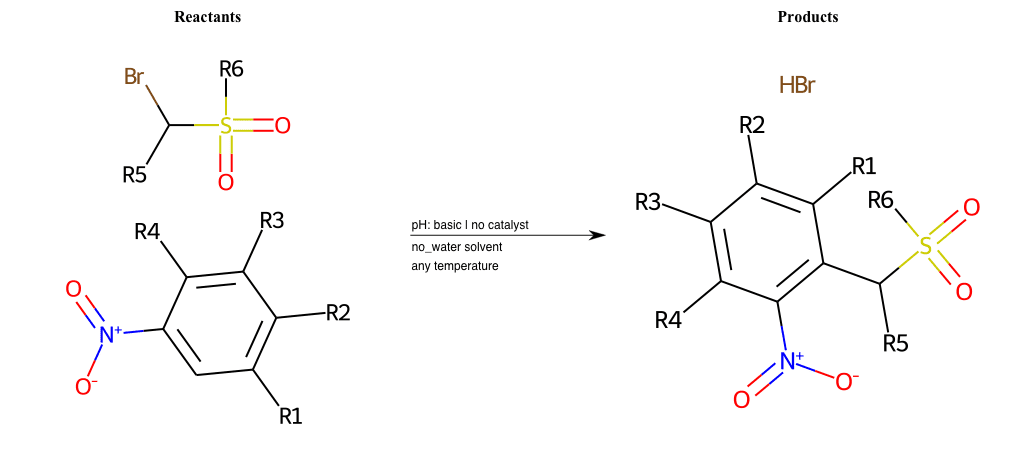
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carboxyl-and-EWG2-Alkane-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
R8 = H, A-Aliphatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
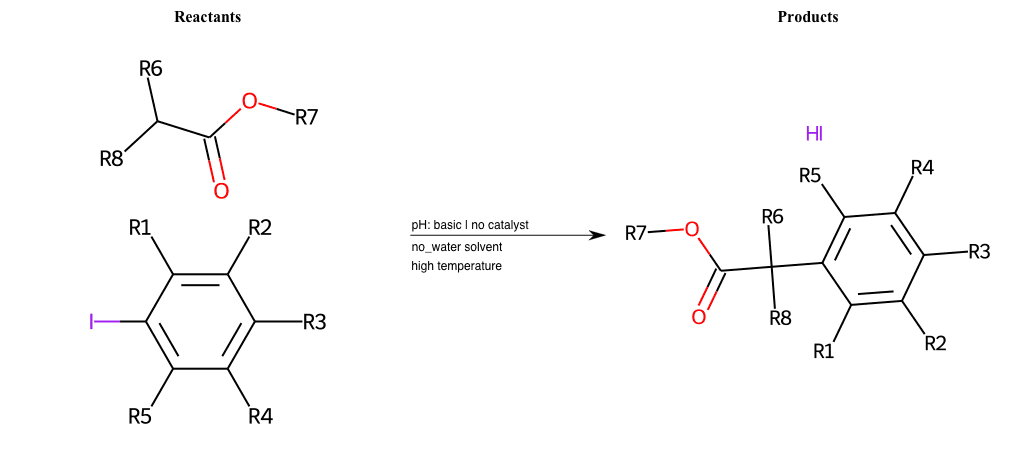
# Substituted-Pyridine-Nucleophilic-Aromatic-Substitution-Lg-Iodine-and-Nu-Thiolate
References:
[0]
Weickgenannt_Jun_12.pdf
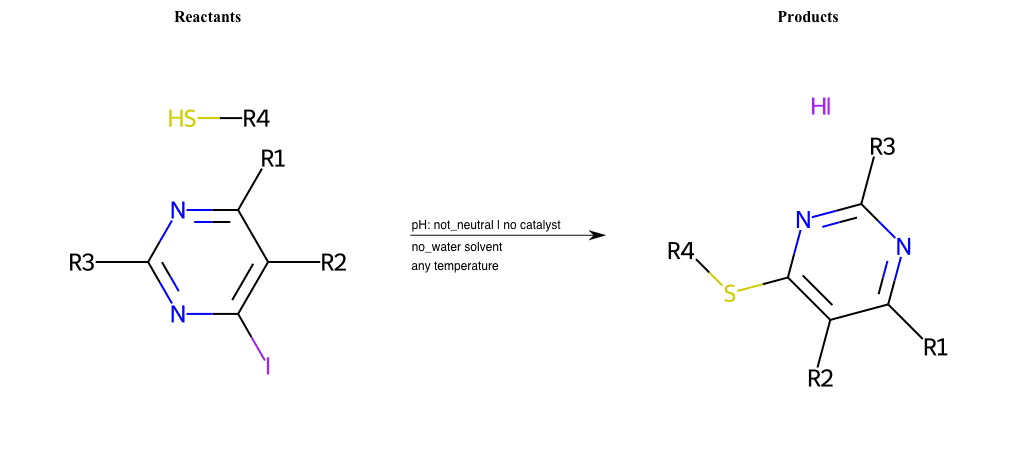
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carbonyl-and-EWG2-Nitrile-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
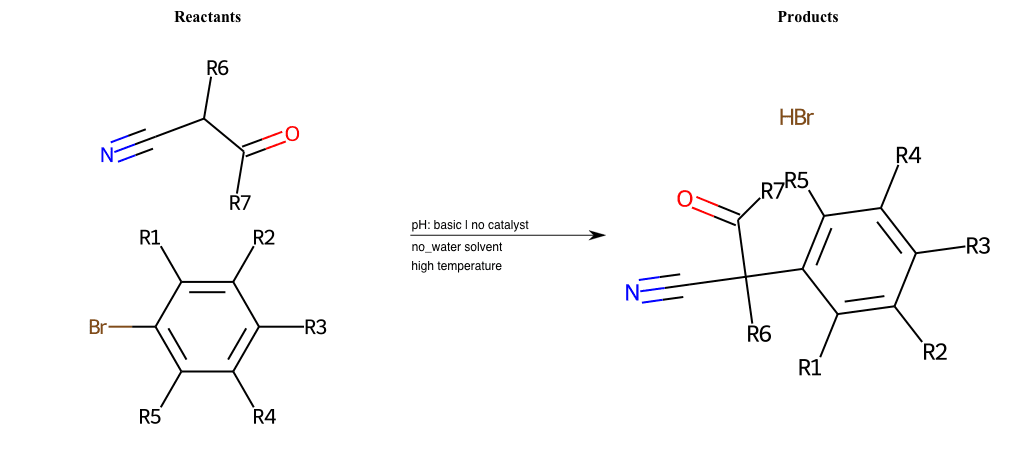
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Nitrile-and-EWG2-Alkane-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = H, A-Aliphatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
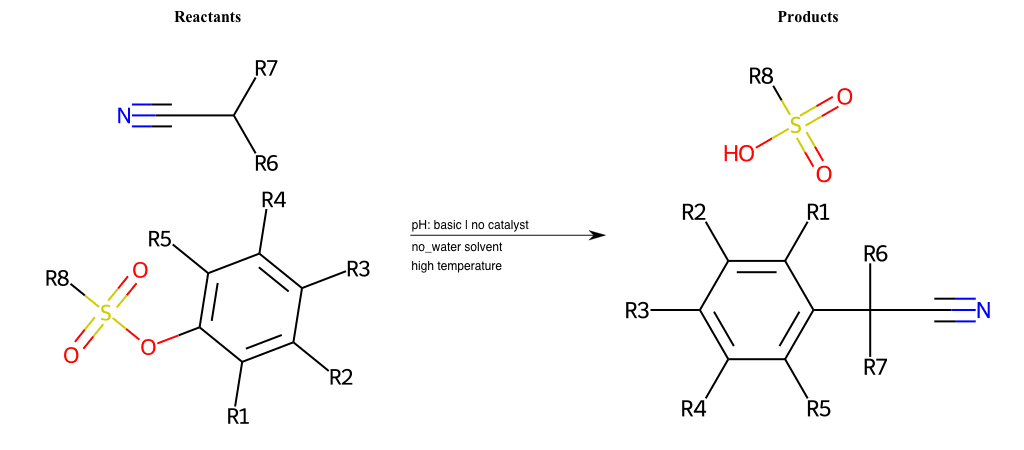
# Vicarious-Nucleophilic-Substitution-Ortho-X-Iodine-and-EWG-Sulfonyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
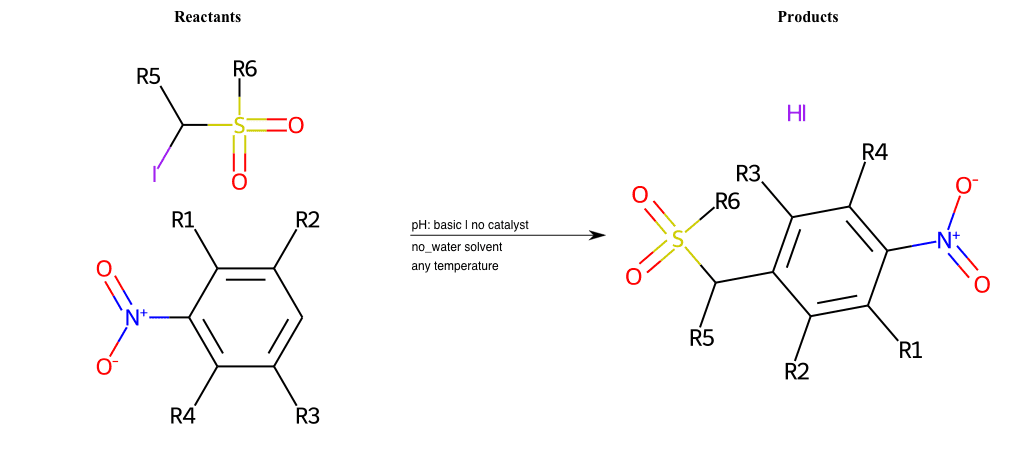
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Phosphonate-and-EWG2-Phosphonate-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R8 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R9 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R10 = A-Aliphatic-Carbon, A-Aromatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
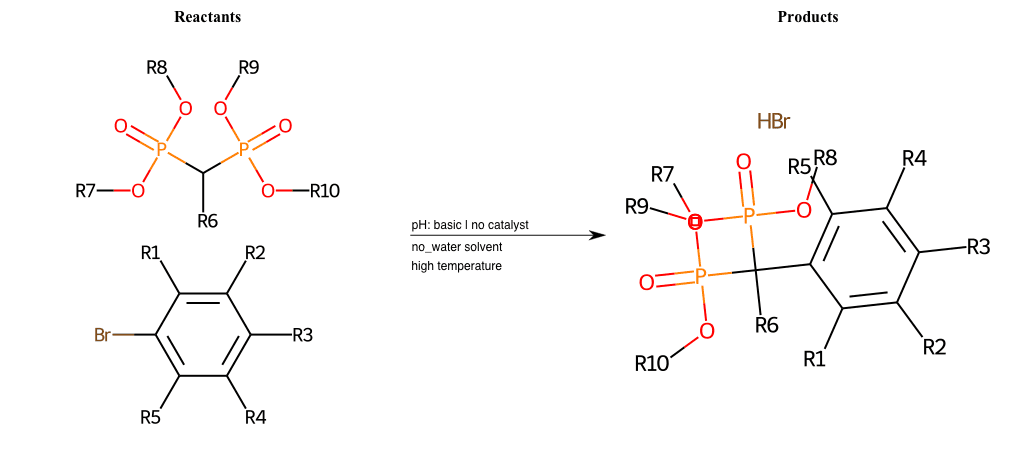
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carbonyl-and-EWG2-Alkane-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
R8 = H, A-Aliphatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
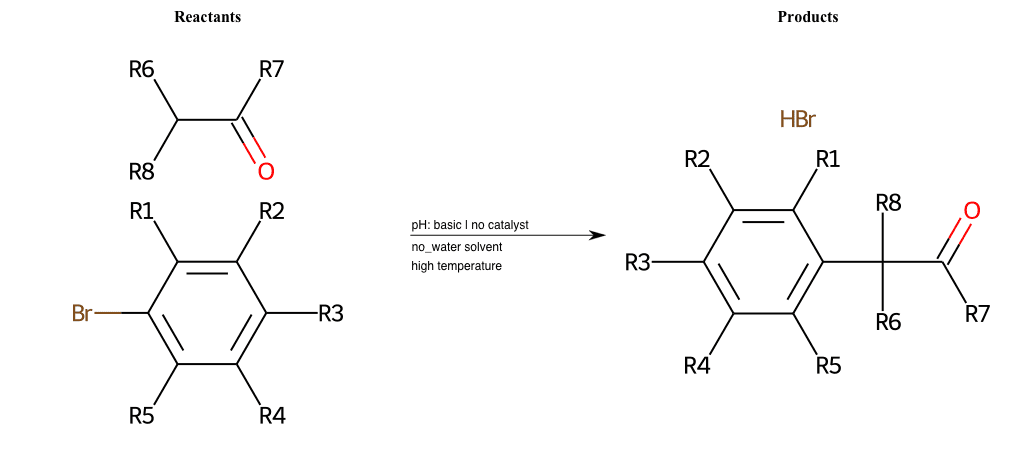
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Nitrile-and-EWG2-Nitrile-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
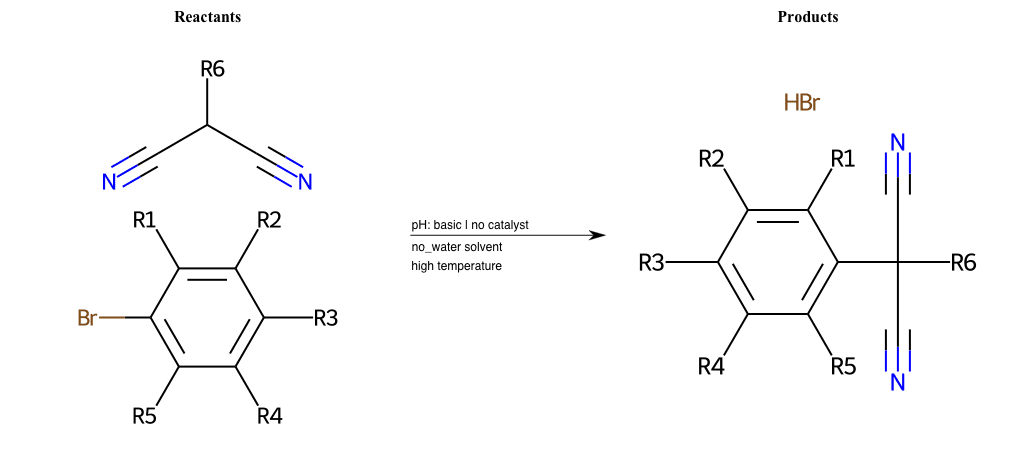
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Nitrile-and-EWG2-Nitrile-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
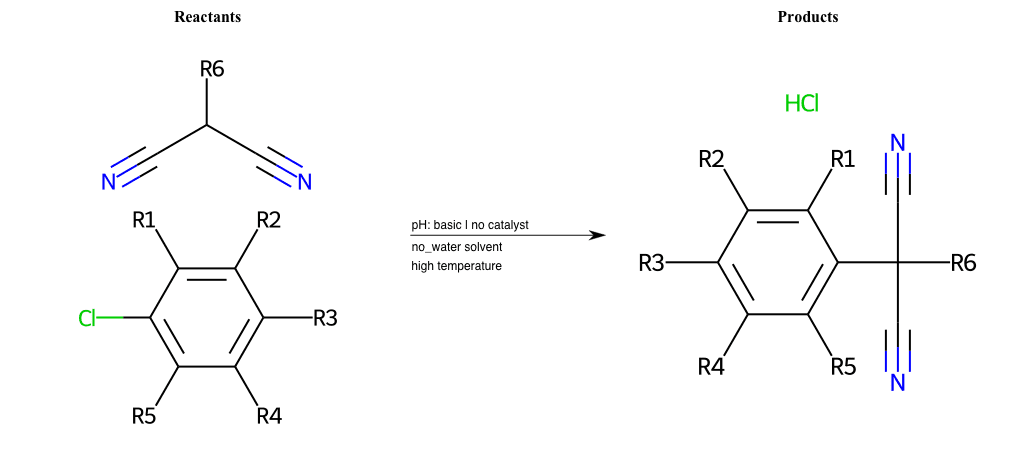
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carboxyl-and-EWG2-Nitrite-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
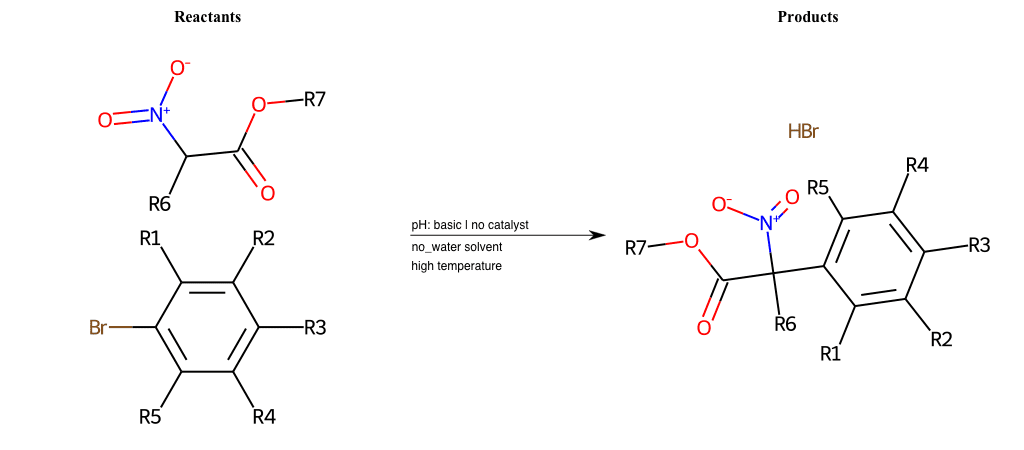
# Substituted-Pyridine-Nucleophilic-Aromatic-Substitution-Lg-Iodine-and-Nu-Amino
References:
[0]
Weickgenannt_Jun_12.pdf
Condition to enforce:
R4 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
R5 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
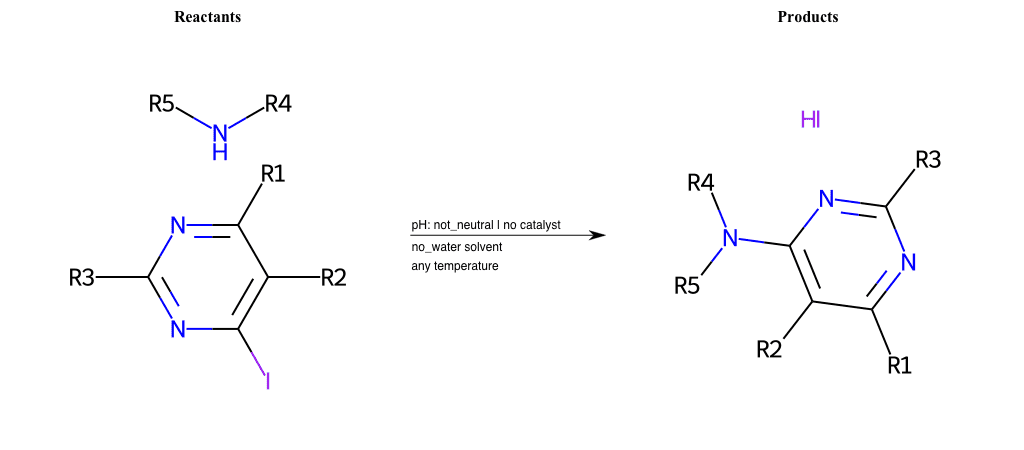
# Vicarious-Nucleophilic-Substitution-Para-X-Iodine-and-EWG-Sulfonyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
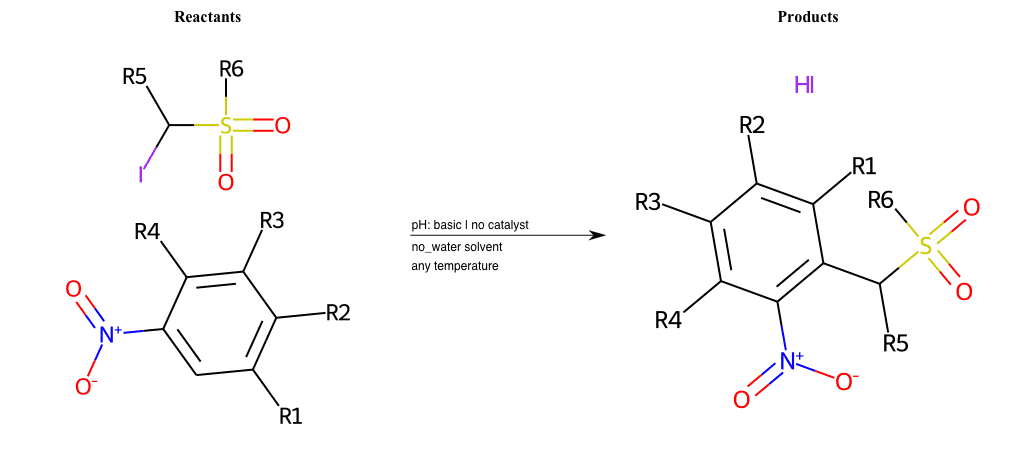
# Nucleophilic-Aromatic-Substitution-Lg-Fluorine-and-Nu-Amino
References:
[0]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[1]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[2]
Nucleophilic Aromatic Substitution - Chemistry Steps
[3]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
[4]
Explain why the trifluoromethyl (CF_3) group is meta directing in electrophilic aromatic substitution. What would you expect CF_3 to be activating or deactivating? Why? Can some one PLEASE help me!!!!! Thanks? | Socratic
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
R7 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
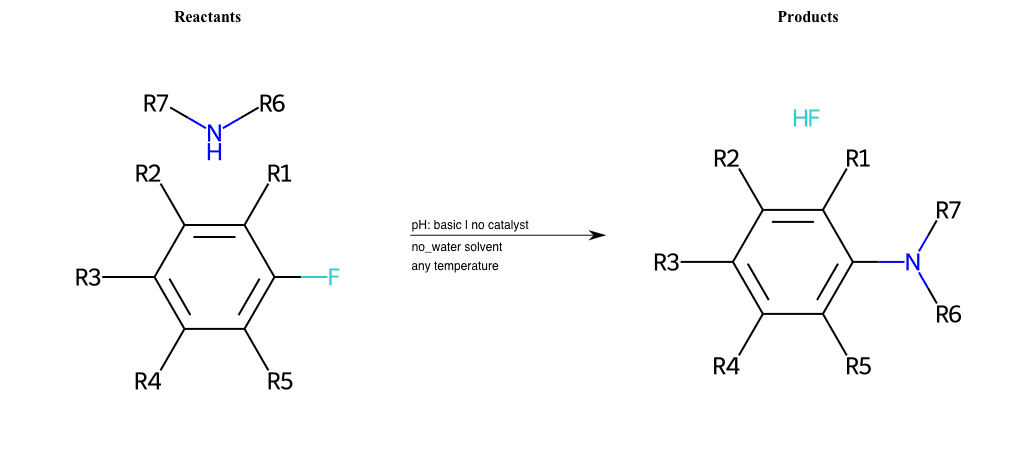
# Vicarious-Nucleophilic-Substitution-Para-X-Sulfonate-and-EWG-Sulfonyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R7 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
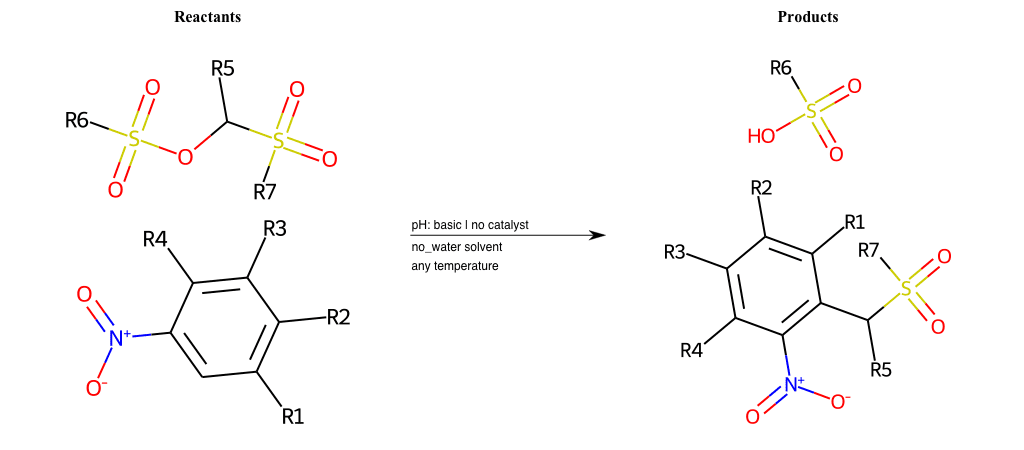
# Nucleophilic-Aromatic-Substitution-Nitric-Acid-Lg-Iodine
References:
[0]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[1]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[2]
Nucleophilic Aromatic Substitution - Chemistry Steps
[3]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
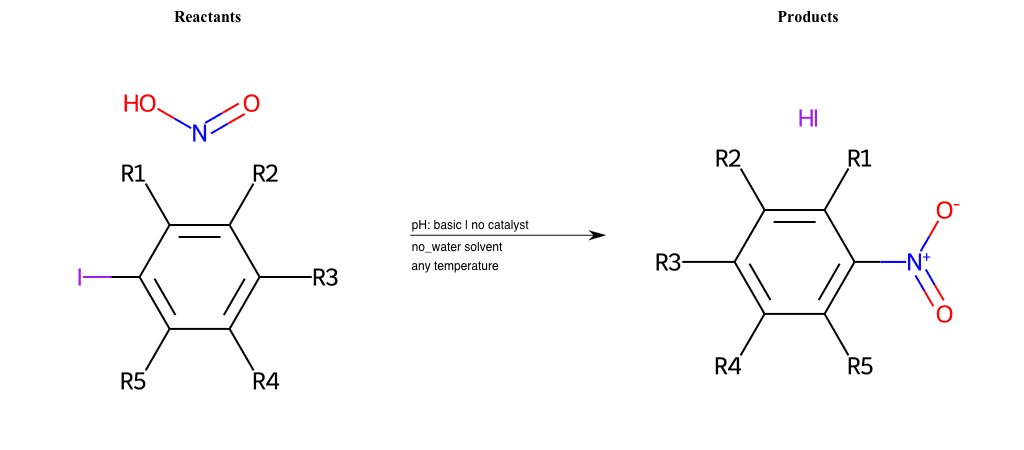
# Substituted-Pyridine-Nucleophilic-Aromatic-Substitution-Lg-Bromine-and-Nu-Hydroxyl
References:
[0]
Weickgenannt_Jun_12.pdf
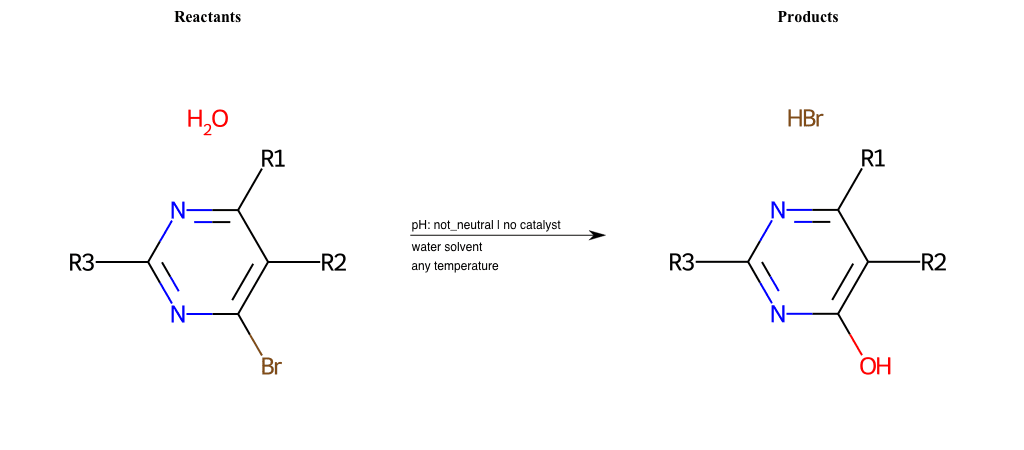
# Vicarious-Nucleophilic-Substitution-Para-X-Thiolate-and-EWG-Carboxyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R7 = A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
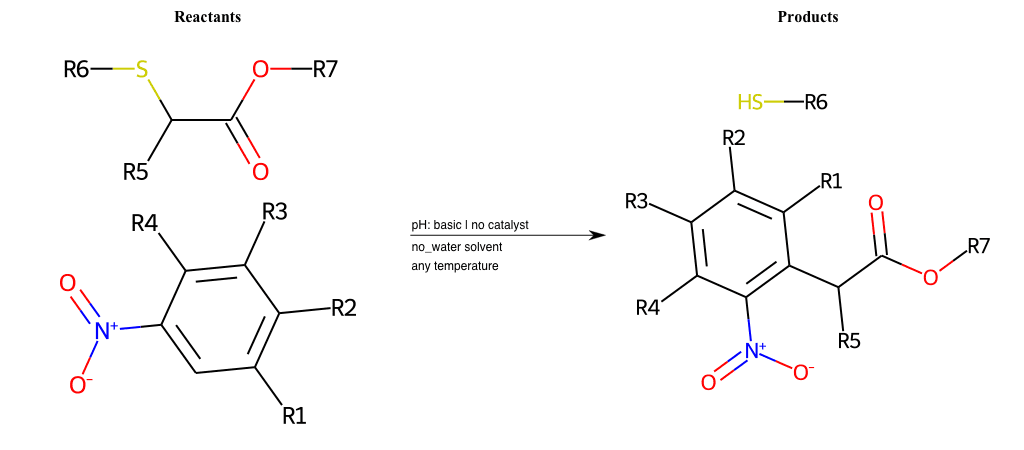
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carbonyl-and-EWG2-Carboxyl-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
R8 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
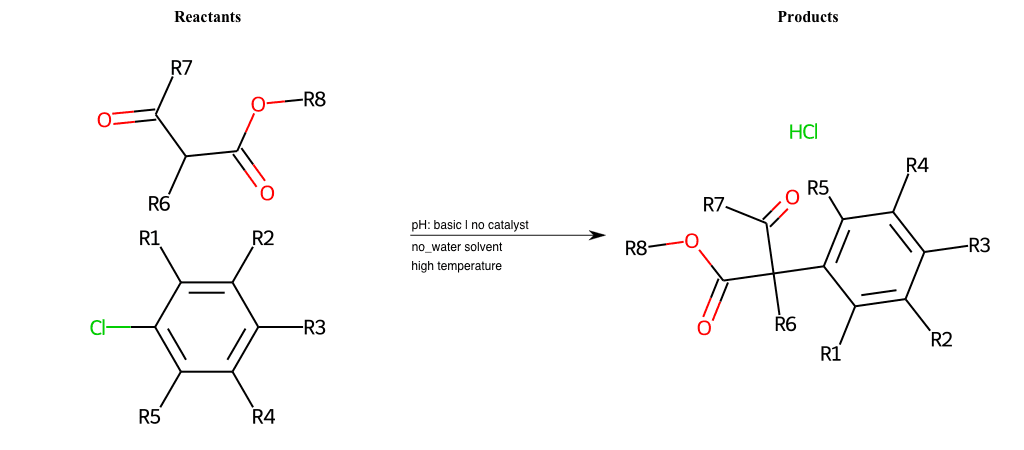
# Vicarious-Nucleophilic-Substitution-Ortho-X-Sulfonate-and-EWG-Sulfonyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R7 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
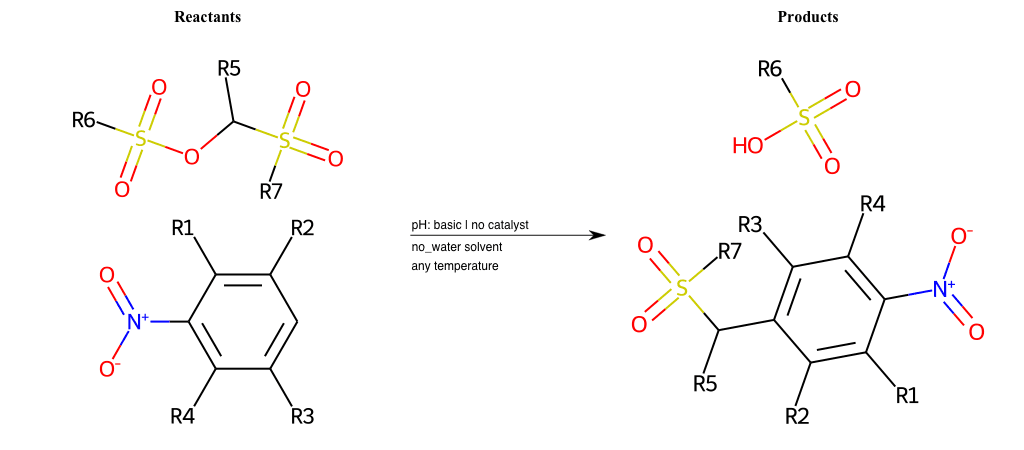
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carboxyl-and-EWG2-Alkane-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
R8 = H, A-Aliphatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
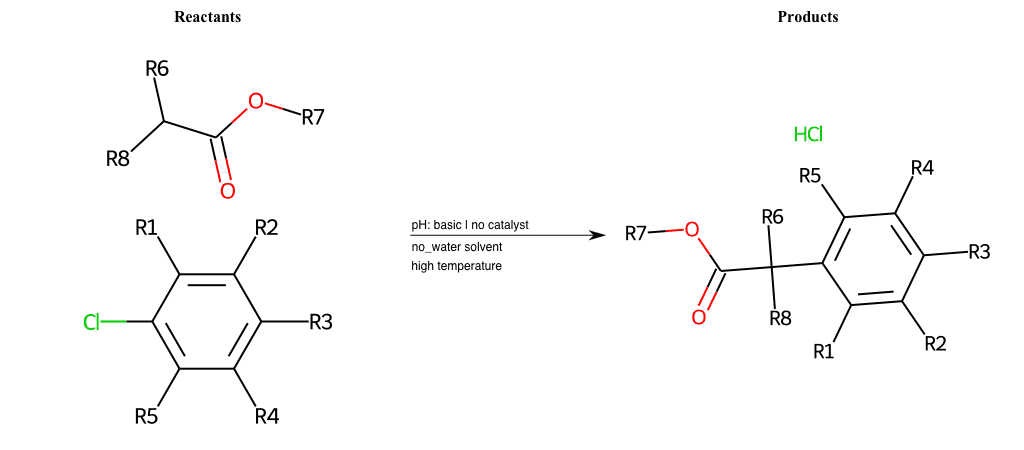
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Nitrite-and-EWG2-Phosphonate-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R8 = A-Aliphatic-Carbon, A-Aromatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
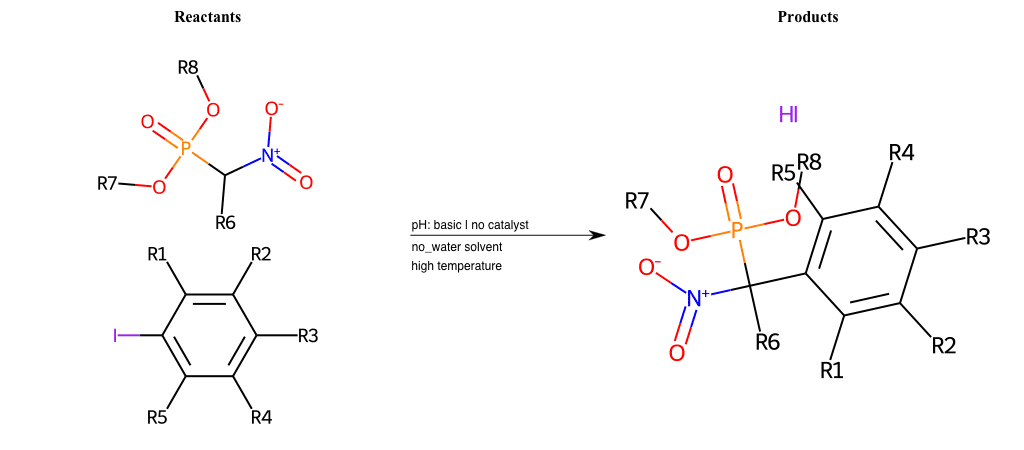
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carboxyl-and-EWG2-Alkane-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
R8 = H, A-Aliphatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
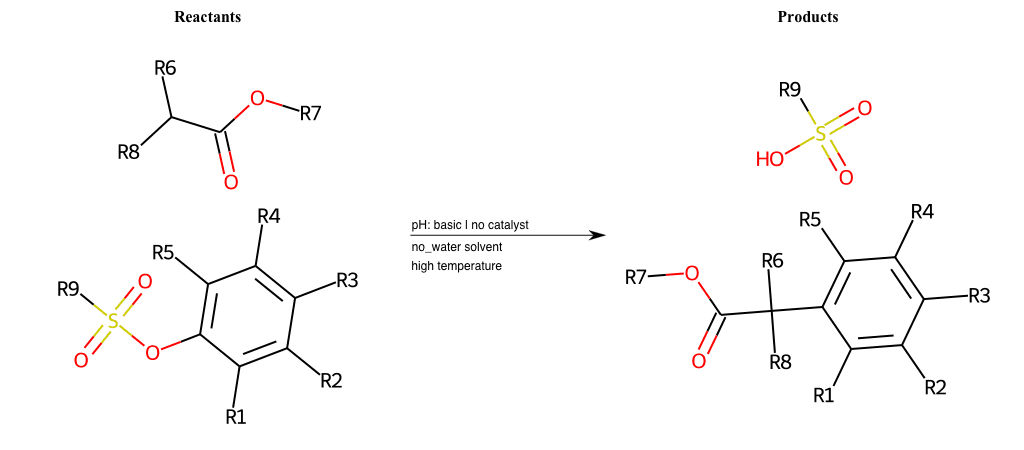
# Vicarious-Nucleophilic-Substitution-Ortho-X-Thiolate-and-EWG-Nitrile
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
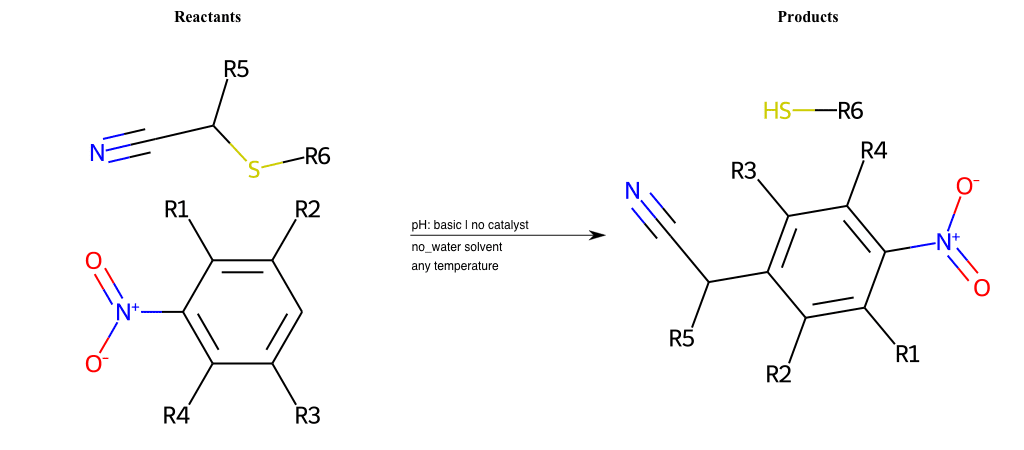
# Nucleophilic-Aromatic-Substitution-Pyridine-Ortho-Lg-Fluorine-and-Nu-Hydroxyl
References:
[0]
organic chemistry - Why does nucleophilic aromatic substitution occur at C-2 and C-4 of pyridine? - Chemistry Stack Exchange
[1]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[2]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[3]
Nucleophilic Aromatic Substitution - Chemistry Steps
[4]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
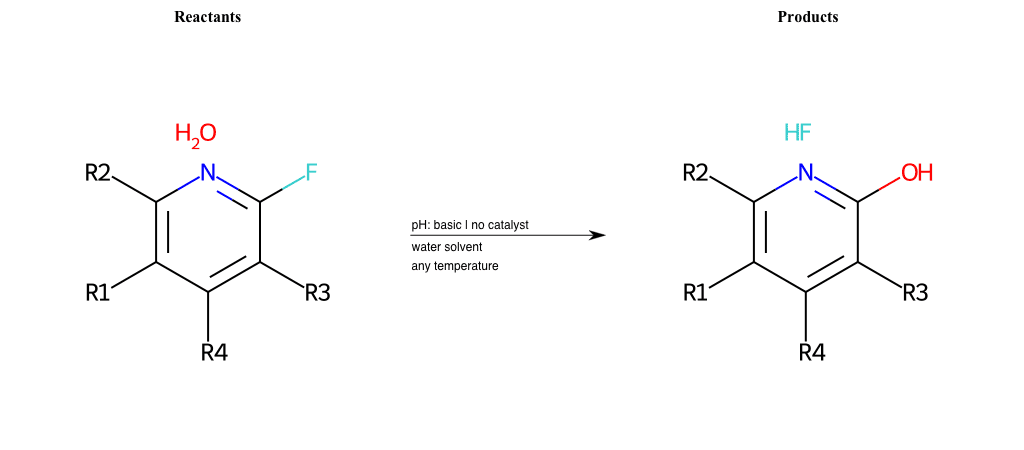
# Nucleophilic-Aromatic-Substitution-Pyridine-Ortho-Lg-Chlorine-and-Nu-Alkoxide
References:
[0]
organic chemistry - Why does nucleophilic aromatic substitution occur at C-2 and C-4 of pyridine? - Chemistry Stack Exchange
[1]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[2]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[3]
Nucleophilic Aromatic Substitution - Chemistry Steps
[4]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
Condition to enforce:
R5 = A-Aliphatic-Carbon, A-Aromatic-Carbon
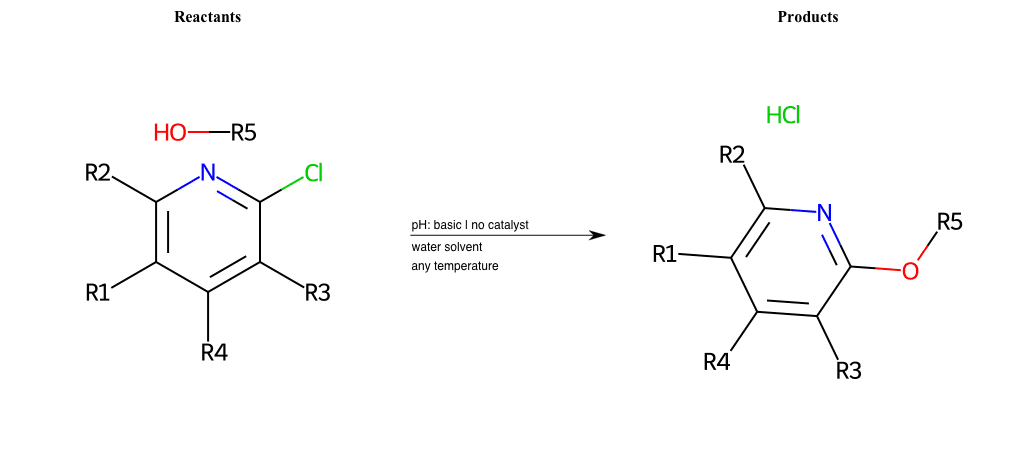
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carbonyl-and-EWG2-Phosphonate-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
R8 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R9 = A-Aliphatic-Carbon, A-Aromatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
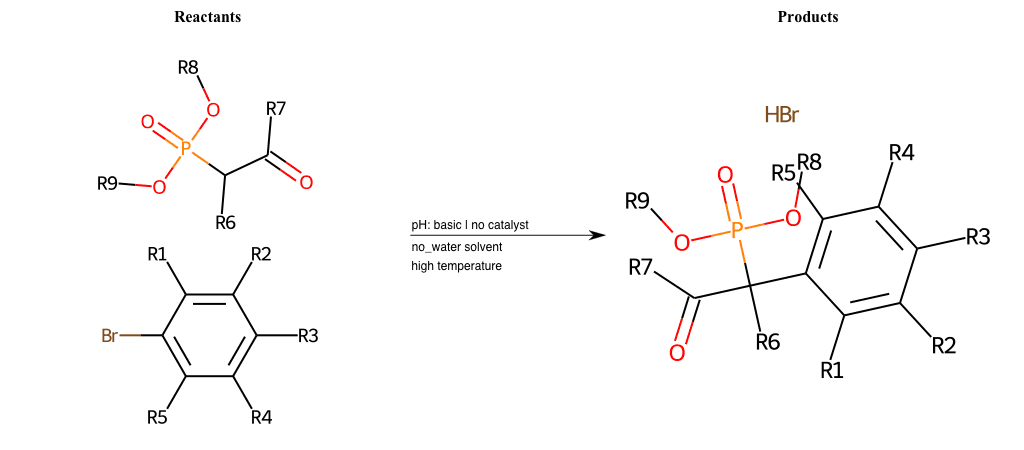
# Vicarious-Nucleophilic-Substitution-Ortho-X-Alkoxide-and-EWG-Carbonyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R7 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
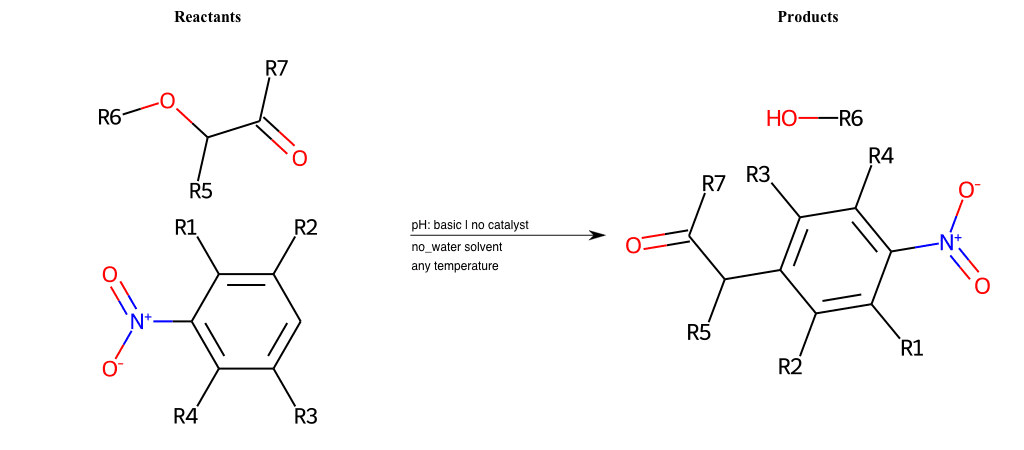
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Nitrile-and-EWG2-Phosphonate-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R8 = A-Aliphatic-Carbon, A-Aromatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
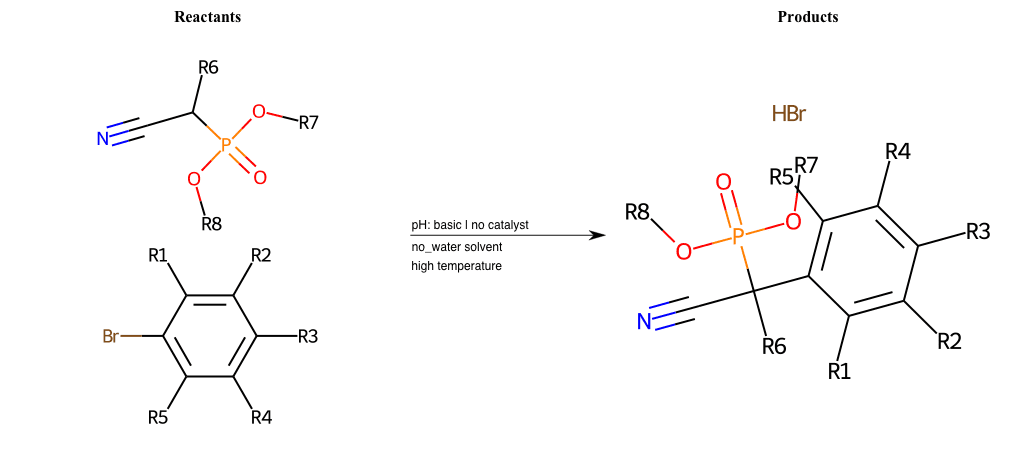
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Nitrite-and-EWG2-Alkane-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = H, A-Aliphatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
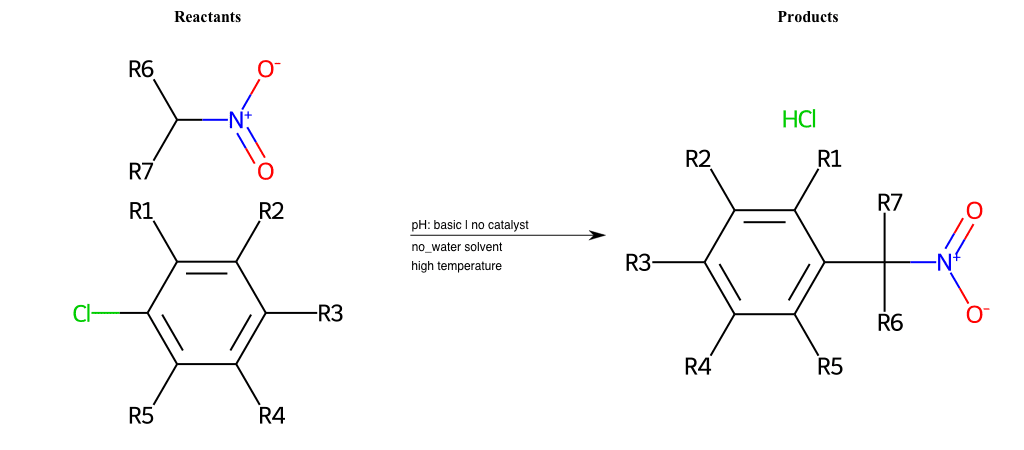
# Vicarious-Nucleophilic-Substitution-Ortho-X-Bromine-and-EWG-Carbonyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
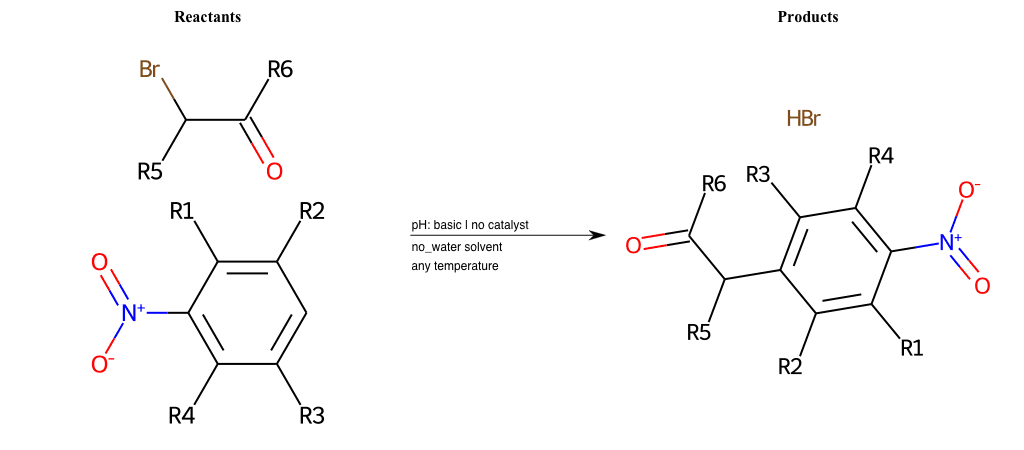
# Nucleophilic-Aromatic-Substitution-Pyridine-Para-Lg-Fluorine-and-Nu-Thiolate
References:
[0]
organic chemistry - Why does nucleophilic aromatic substitution occur at C-2 and C-4 of pyridine? - Chemistry Stack Exchange
[1]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[2]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[3]
Nucleophilic Aromatic Substitution - Chemistry Steps
[4]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
Condition to enforce:
R5 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
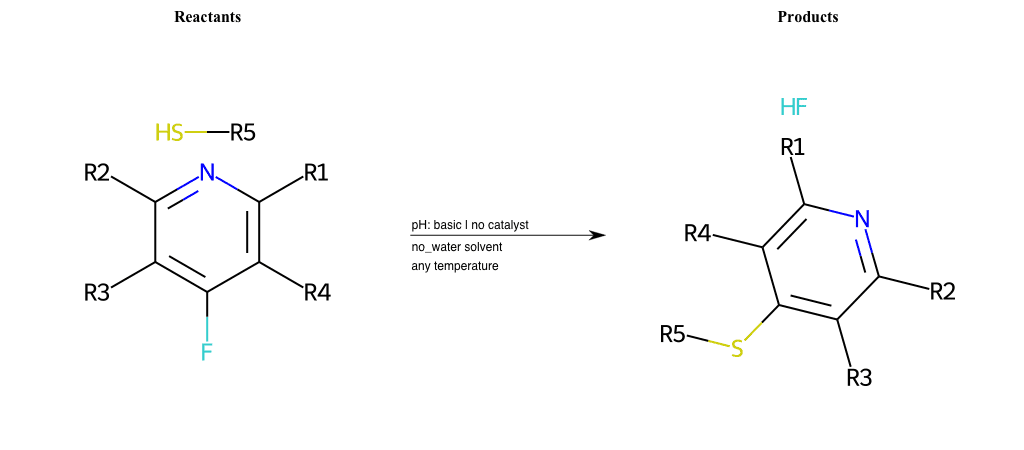
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carboxyl-and-EWG2-Nitrile-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
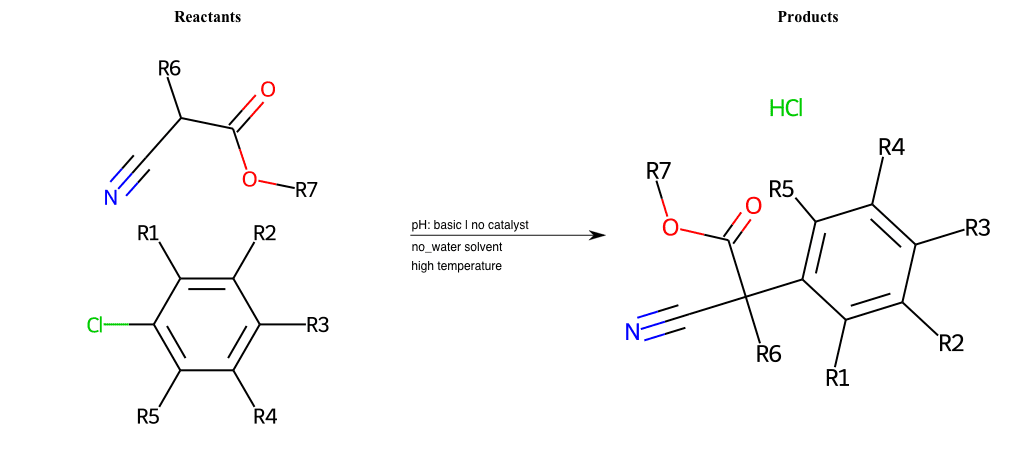
# Nucleophilic-Aromatic-Substitution-Pyridine-Para-Lg-Iodine-and-Nu-Alkoxide
References:
[0]
organic chemistry - Why does nucleophilic aromatic substitution occur at C-2 and C-4 of pyridine? - Chemistry Stack Exchange
[1]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[2]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[3]
Nucleophilic Aromatic Substitution - Chemistry Steps
[4]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
Condition to enforce:
R5 = A-Aliphatic-Carbon, A-Aromatic-Carbon
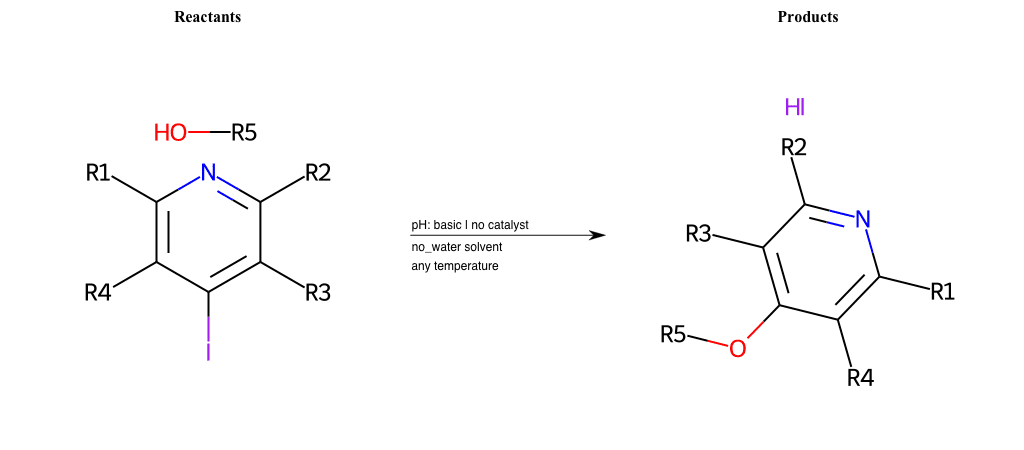
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carbonyl-and-EWG2-Alkane-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
R8 = H, A-Aliphatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
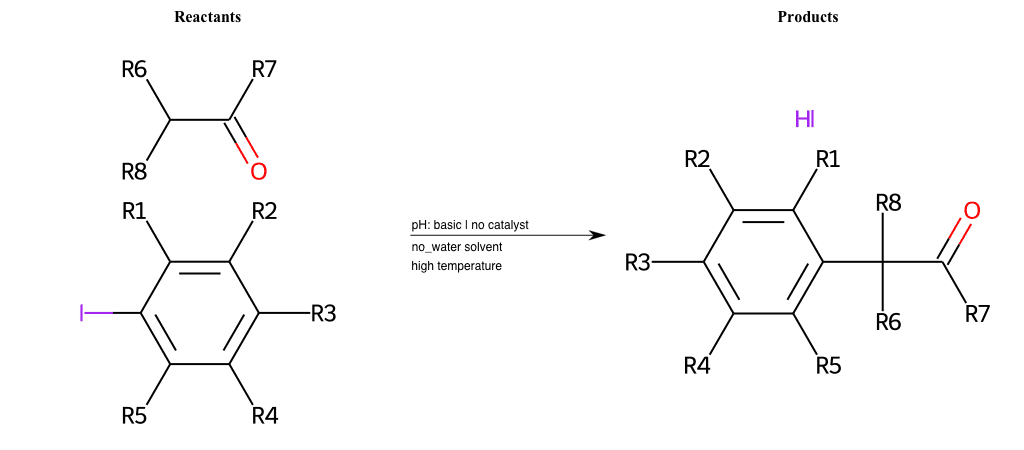
# Substituted-Pyridine-Nucleophilic-Aromatic-Substitution-Lg-Chlorine-and-Nu-Hydroxyl
References:
[0]
Weickgenannt_Jun_12.pdf
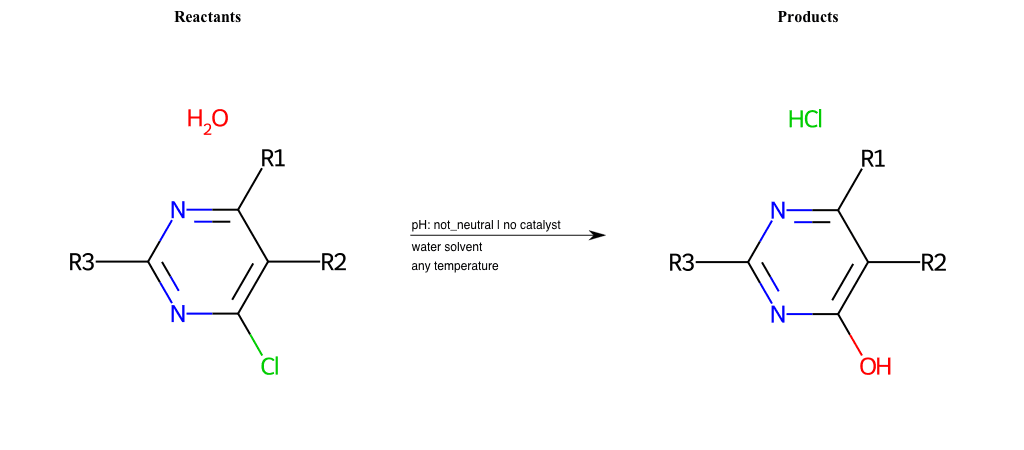
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carbonyl-and-EWG2-Phosphonate-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
R8 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R9 = A-Aliphatic-Carbon, A-Aromatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
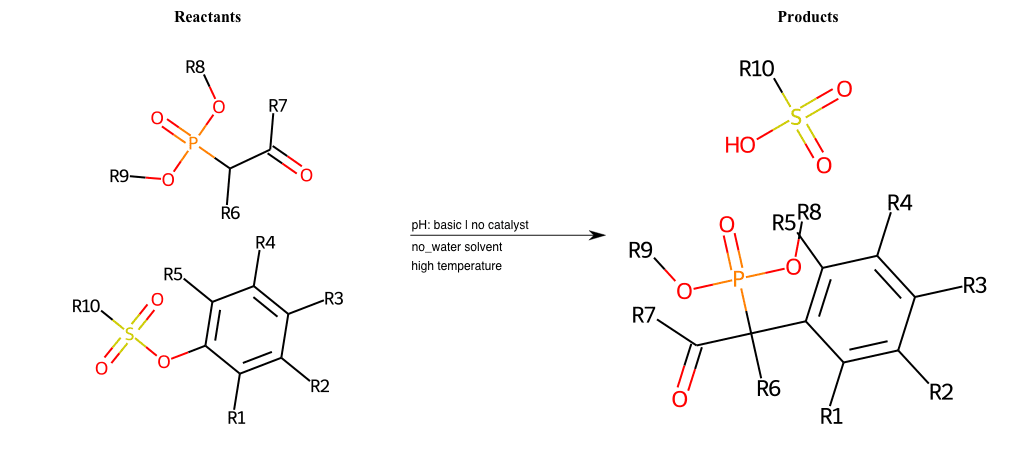
# Vicarious-Nucleophilic-Substitution-Para-X-Alkoxide-and-EWG-Sulfonyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R7 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
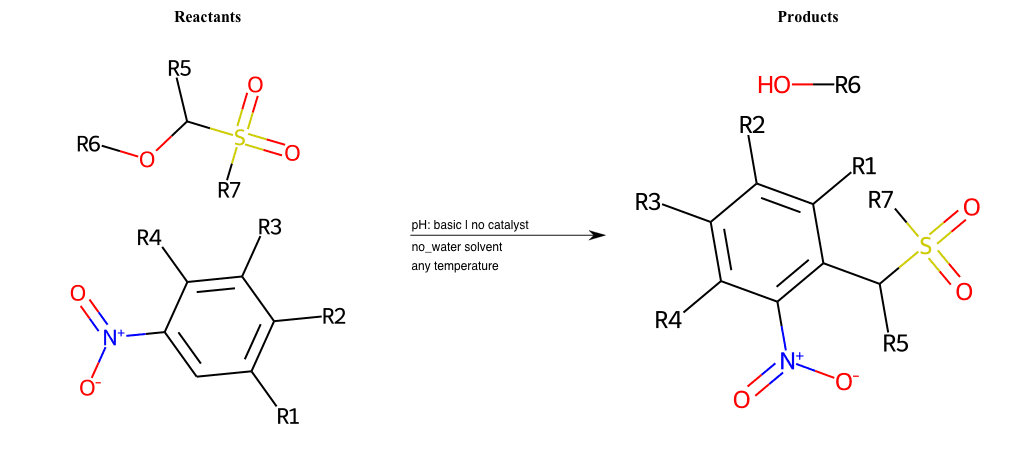
# Substituted-Pyridine-Nucleophilic-Aromatic-Substitution-Lg-Chlorine-and-Nu-Thiolate
References:
[0]
Weickgenannt_Jun_12.pdf
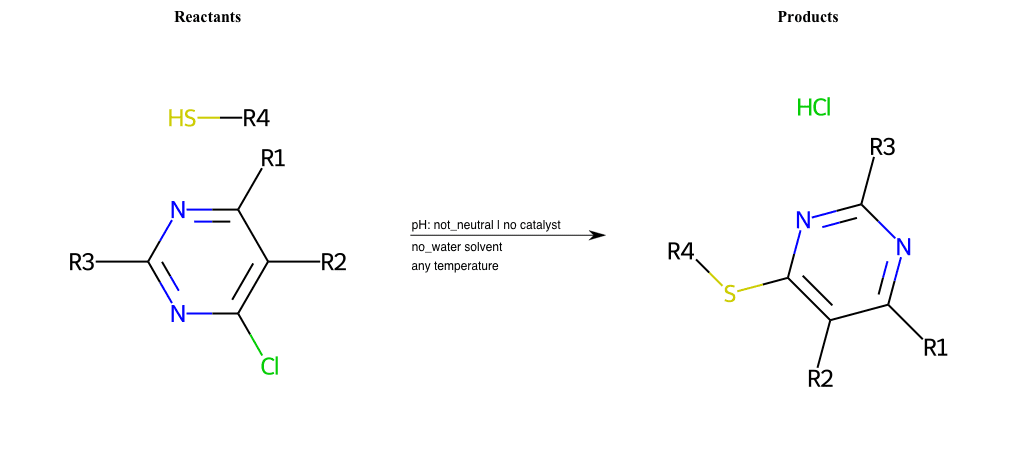
# Nucleophilic-Aromatic-Substitution-Lg-Iodine-and-Nu-Alkoxide
References:
[0]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[1]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[2]
Nucleophilic Aromatic Substitution - Chemistry Steps
[3]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
[4]
Explain why the trifluoromethyl (CF_3) group is meta directing in electrophilic aromatic substitution. What would you expect CF_3 to be activating or deactivating? Why? Can some one PLEASE help me!!!!! Thanks? | Socratic
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = A-Aliphatic-Carbon, A-Aromatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
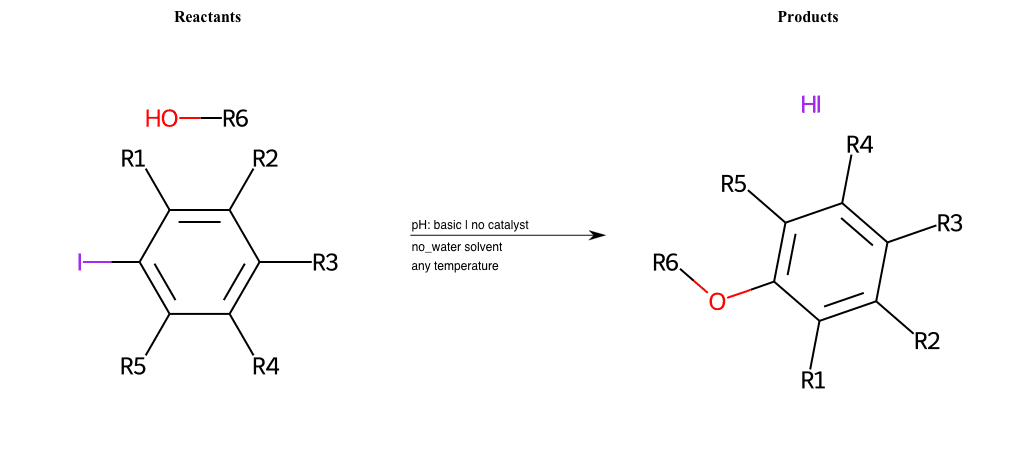
# Vicarious-Nucleophilic-Substitution-Ortho-X-Alkoxide-and-EWG-Carboxyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R7 = A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
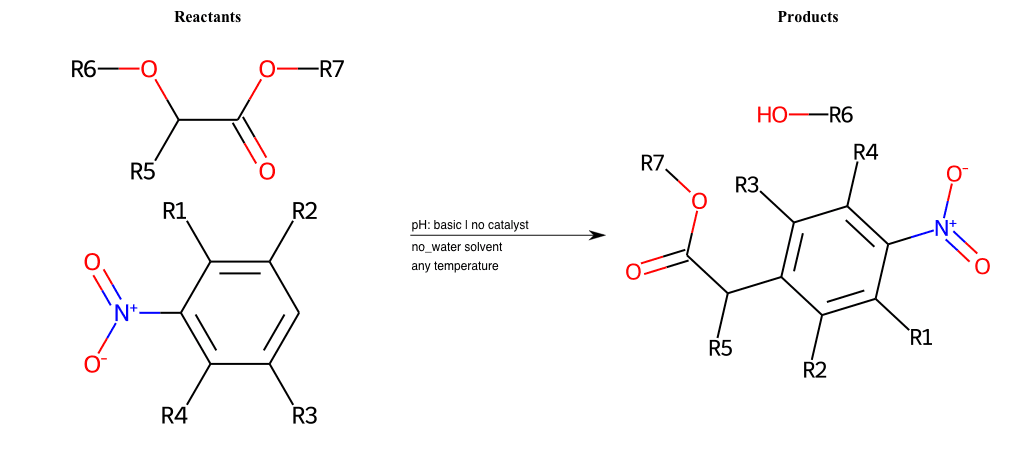
# Nucleophilic-Aromatic-Substitution-Lg-Iodine-and-Nu-Amino
References:
[0]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[1]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[2]
Nucleophilic Aromatic Substitution - Chemistry Steps
[3]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
[4]
Explain why the trifluoromethyl (CF_3) group is meta directing in electrophilic aromatic substitution. What would you expect CF_3 to be activating or deactivating? Why? Can some one PLEASE help me!!!!! Thanks? | Socratic
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
R7 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
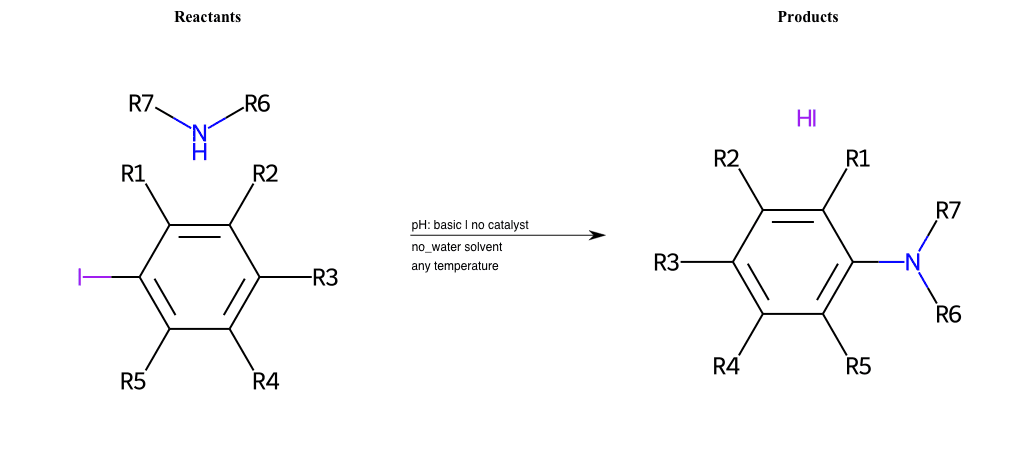
# Substituted-Pyridine-Nucleophilic-Aromatic-Substitution-Lg-Bromine-and-Nu-Thiolate
References:
[0]
Weickgenannt_Jun_12.pdf
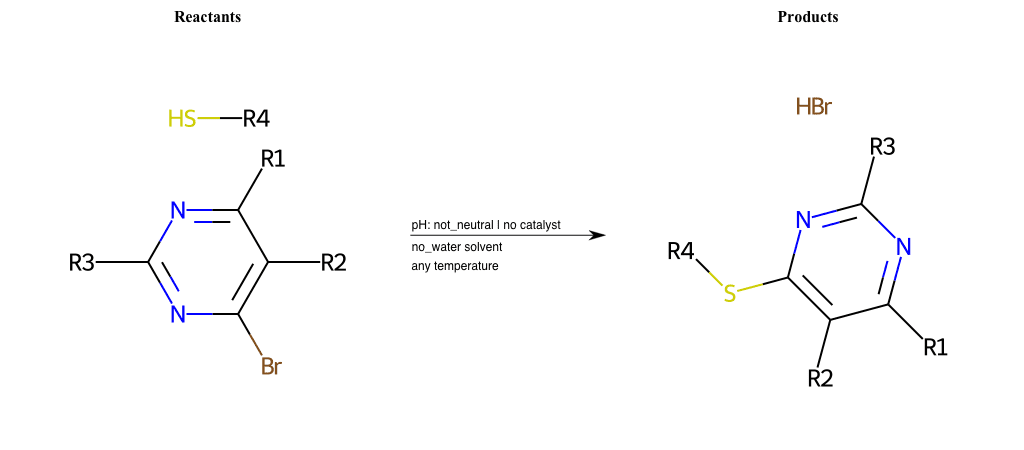
# Nucleophilic-Aromatic-Substitution-Lg-Fluorine-and-Nu-Thiolate
References:
[0]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[1]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[2]
Nucleophilic Aromatic Substitution - Chemistry Steps
[3]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
[4]
Explain why the trifluoromethyl (CF_3) group is meta directing in electrophilic aromatic substitution. What would you expect CF_3 to be activating or deactivating? Why? Can some one PLEASE help me!!!!! Thanks? | Socratic
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
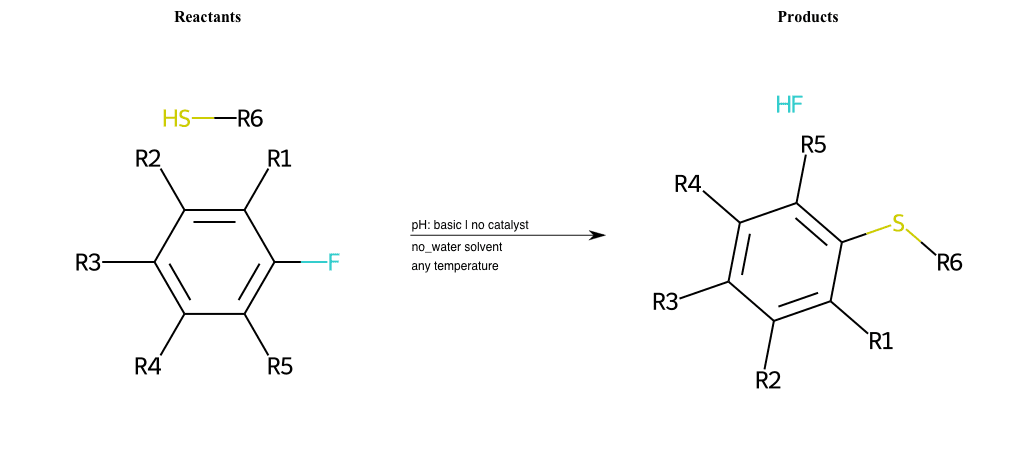
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carbonyl-and-EWG2-Nitrite-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
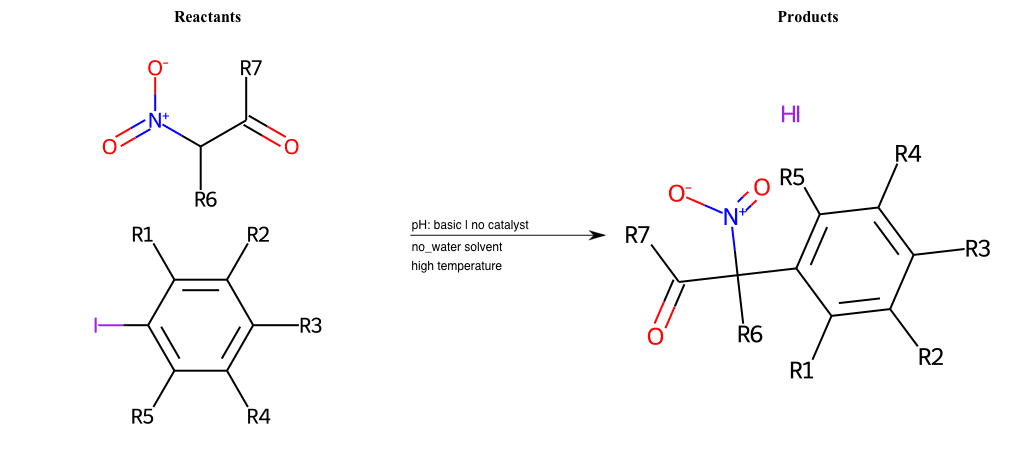
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Phosphonate-and-EWG2-Phosphonate-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R8 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R9 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R10 = A-Aliphatic-Carbon, A-Aromatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
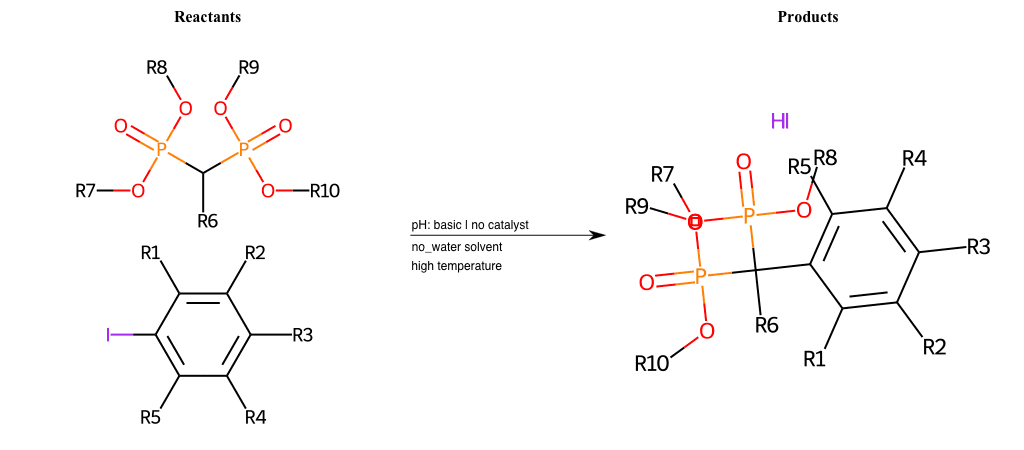
# Nucleophilic-Aromatic-Substitution-Pyridine-Ortho-Lg-Bromine-and-Nu-Thiolate
References:
[0]
organic chemistry - Why does nucleophilic aromatic substitution occur at C-2 and C-4 of pyridine? - Chemistry Stack Exchange
[1]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[2]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[3]
Nucleophilic Aromatic Substitution - Chemistry Steps
[4]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
Condition to enforce:
R5 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
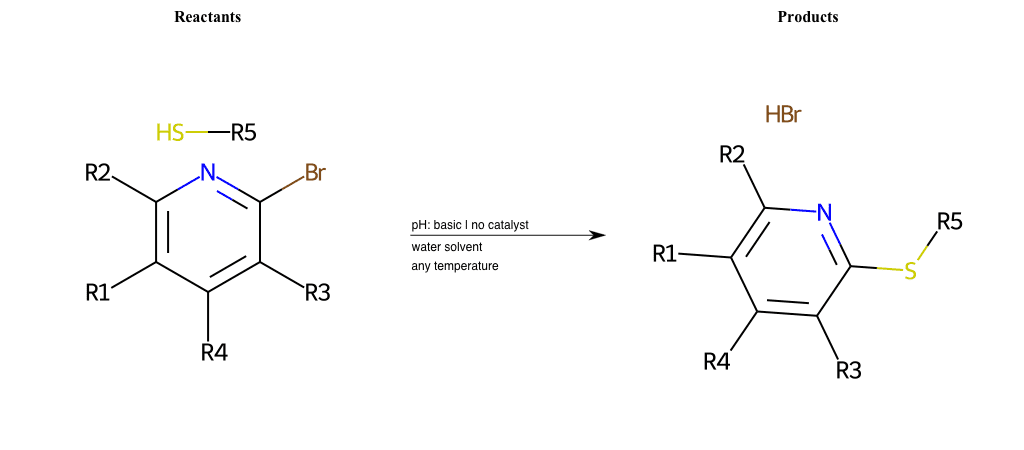
# Nucleophilic-Aromatic-Substitution-Pyridine-Ortho-Lg-Fluorine-and-Nu-Thiolate
References:
[0]
organic chemistry - Why does nucleophilic aromatic substitution occur at C-2 and C-4 of pyridine? - Chemistry Stack Exchange
[1]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[2]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[3]
Nucleophilic Aromatic Substitution - Chemistry Steps
[4]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
Condition to enforce:
R5 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
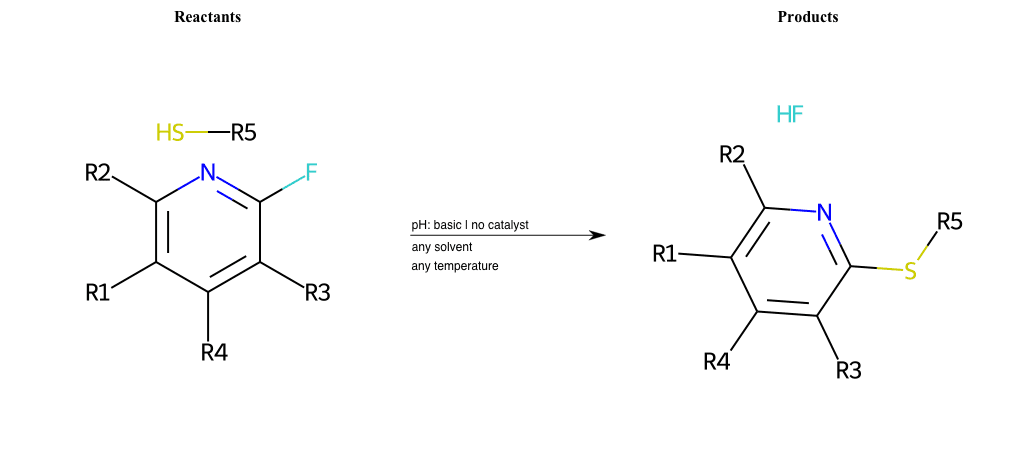
# Substituted-Pyridine-Nucleophilic-Aromatic-Substitution-Lg-Iodine-and-Nu-Hydroxyl
References:
[0]
Weickgenannt_Jun_12.pdf
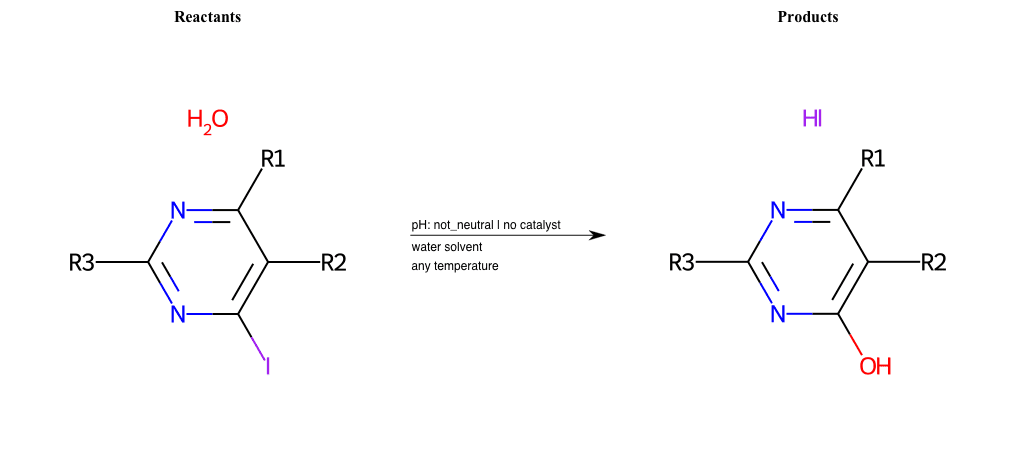
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Nitrile-and-EWG2-Nitrile-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
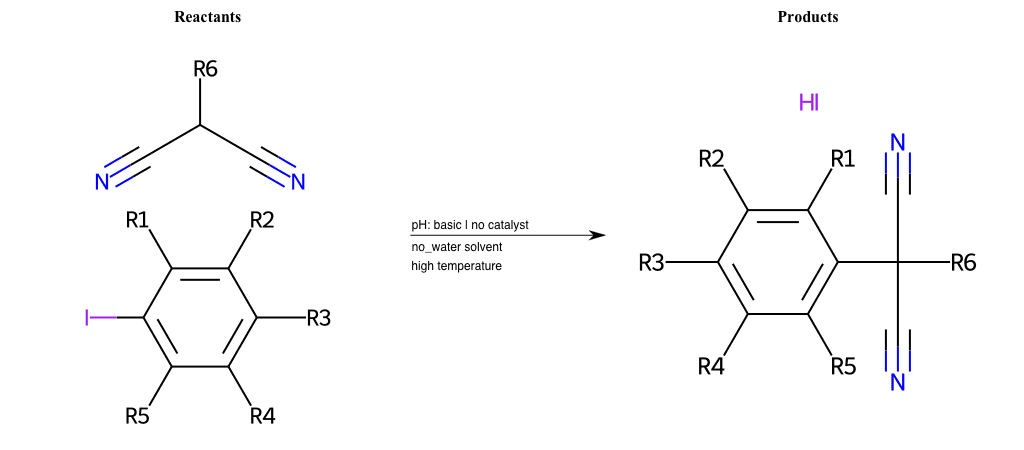
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Nitrile-and-EWG2-Phosphonate-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R8 = A-Aliphatic-Carbon, A-Aromatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
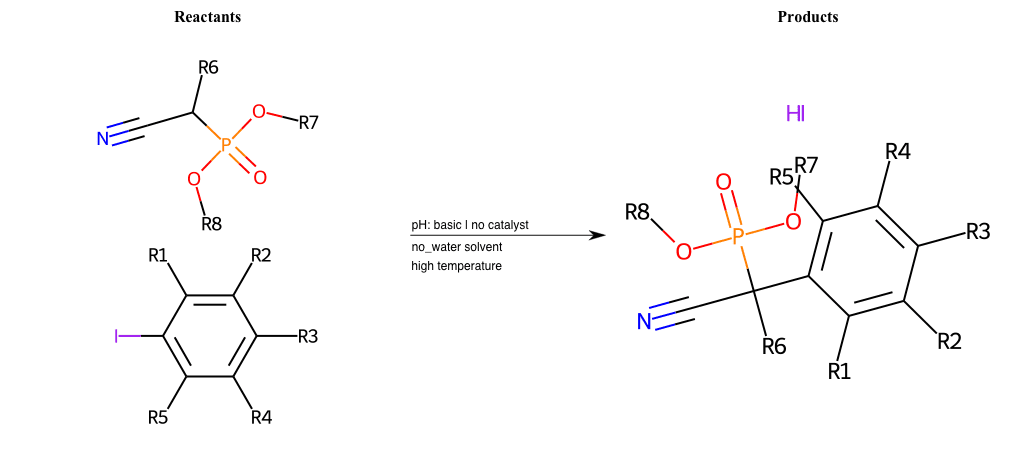
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carboxyl-and-EWG2-Phosphonate-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
R8 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R9 = A-Aliphatic-Carbon, A-Aromatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
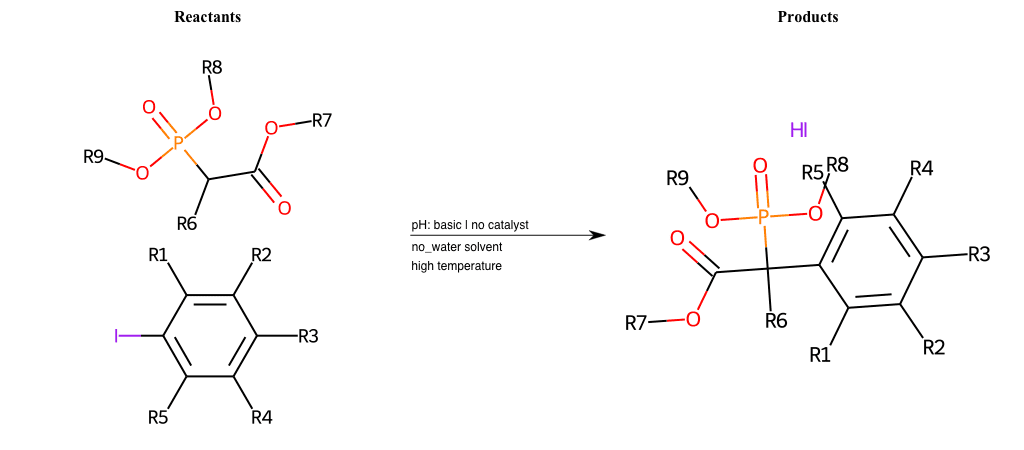
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Nitrile-and-EWG2-Alkane-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = H, A-Aliphatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
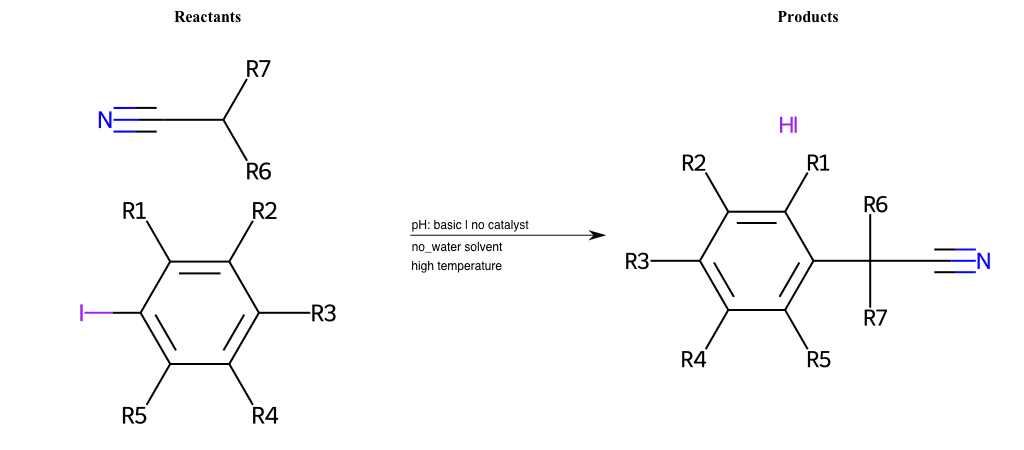
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Nitrile-and-EWG2-Nitrite-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
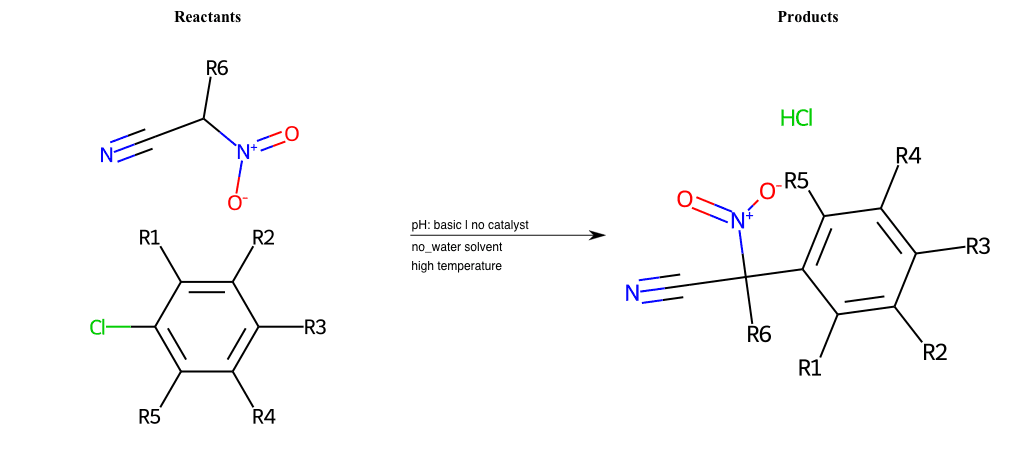
# Nucleophilic-Aromatic-Substitution-Pyridine-Ortho-Lg-Iodine-and-Nu-Thiolate
References:
[0]
organic chemistry - Why does nucleophilic aromatic substitution occur at C-2 and C-4 of pyridine? - Chemistry Stack Exchange
[1]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[2]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[3]
Nucleophilic Aromatic Substitution - Chemistry Steps
[4]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
Condition to enforce:
R5 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
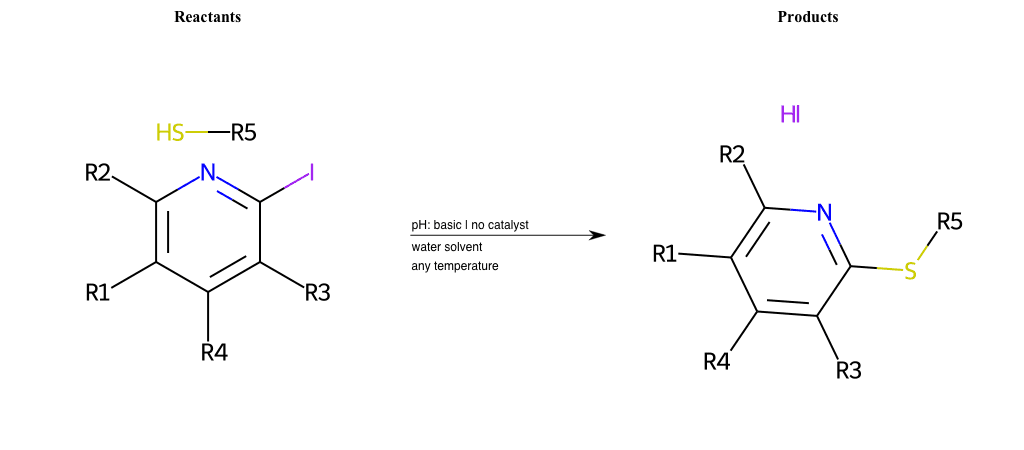
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carbonyl-and-EWG2-Carbonyl-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
R8 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
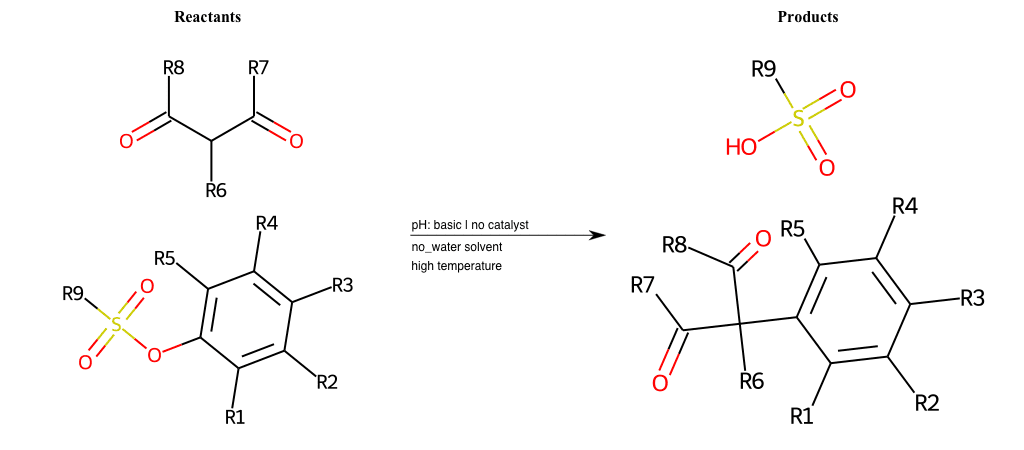
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Nitrite-and-EWG2-Phosphonate-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R8 = A-Aliphatic-Carbon, A-Aromatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
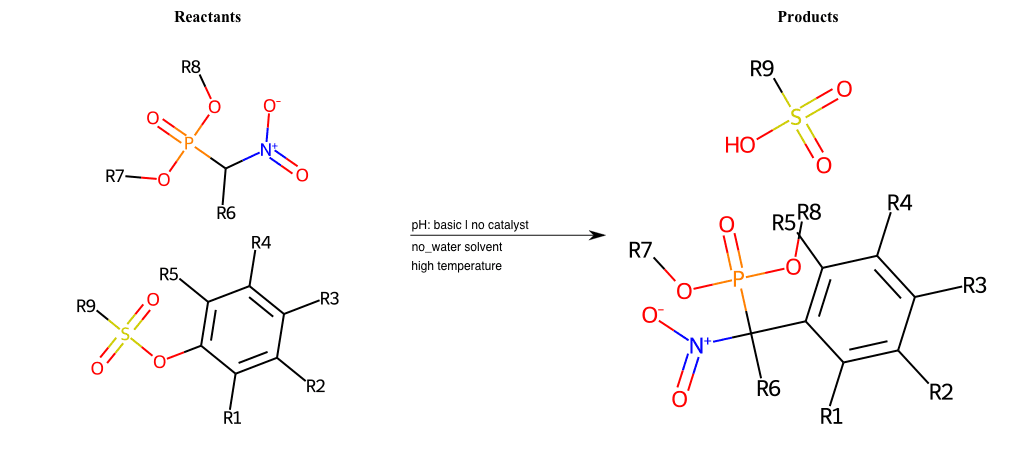
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Nitrite-and-EWG2-Nitrite-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
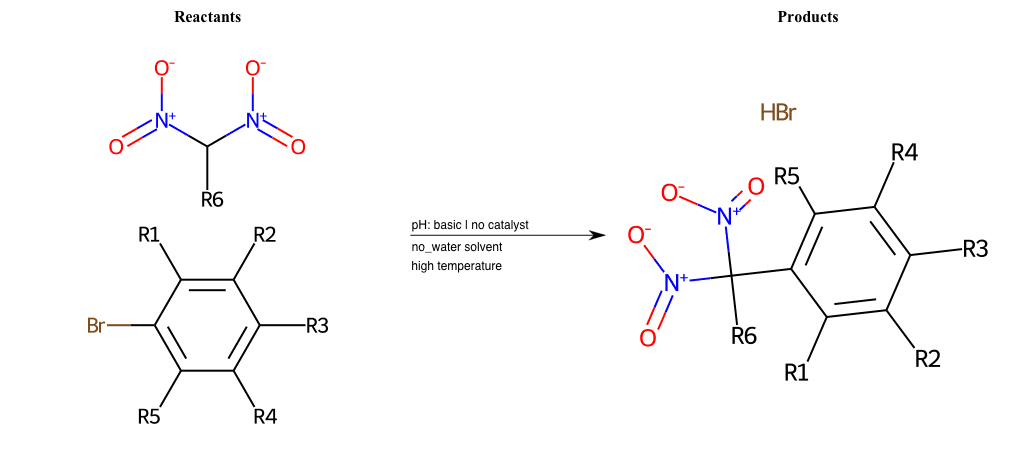
# Vicarious-Nucleophilic-Substitution-Para-X-Chlorine-and-EWG-Carbonyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
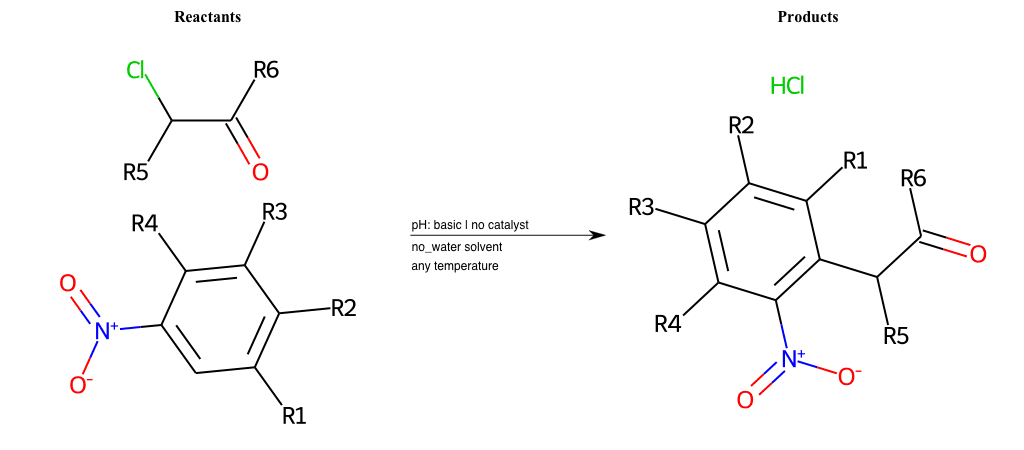
# Vicarious-Nucleophilic-Substitution-Ortho-X-Sulfonate-and-EWG-Nitrile
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
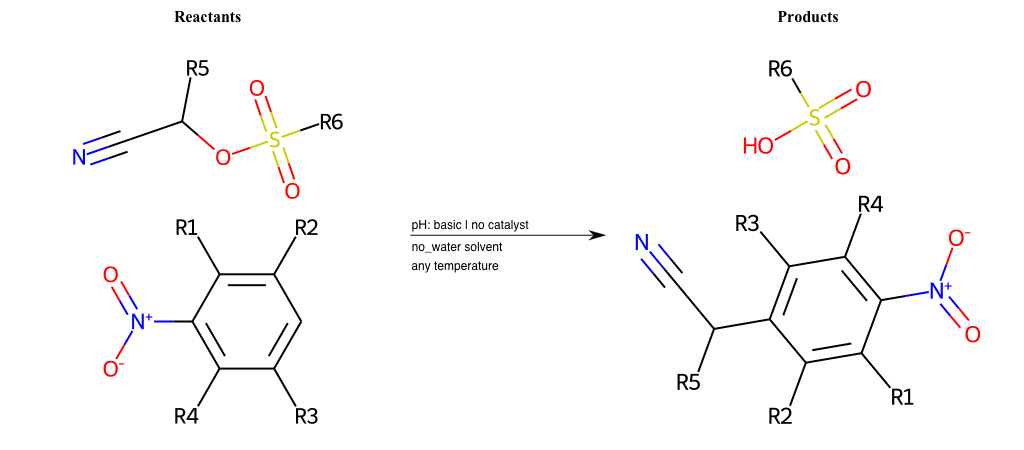
# Vicarious-Nucleophilic-Substitution-Para-X-Chlorine-and-EWG-Carboxyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
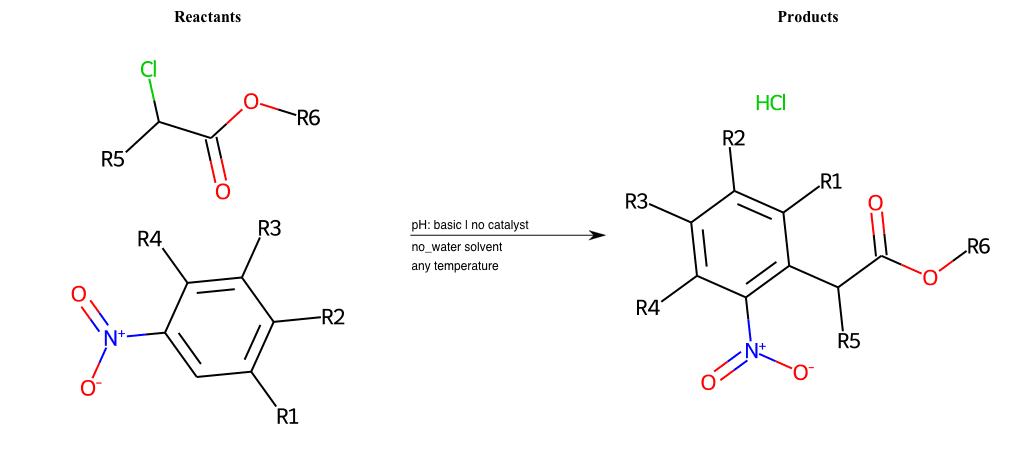
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carboxyl-and-EWG2-Nitrile-Lg-Iodine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
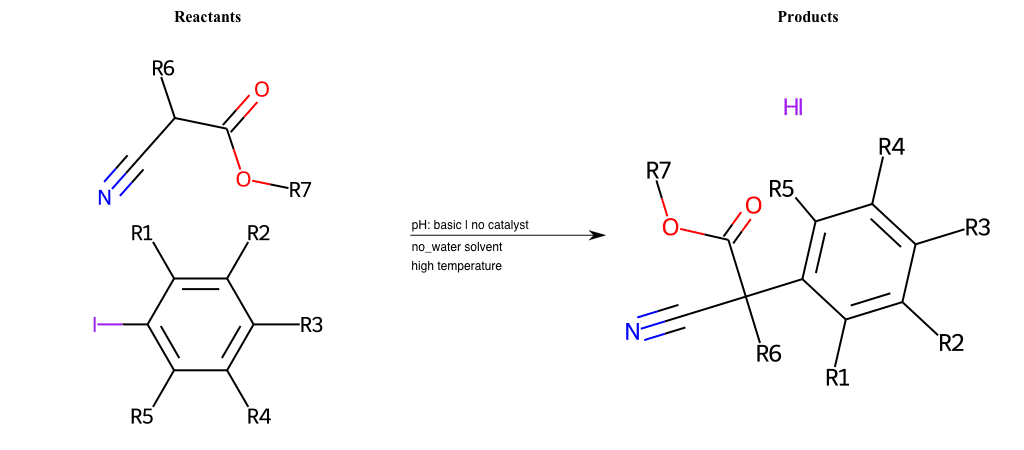
# Nucleophilic-Aromatic-Substitution-Lg-Bromine-and-Nu-Hydroxyl
References:
[0]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[1]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[2]
Nucleophilic Aromatic Substitution - Chemistry Steps
[3]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
[4]
Explain why the trifluoromethyl (CF_3) group is meta directing in electrophilic aromatic substitution. What would you expect CF_3 to be activating or deactivating? Why? Can some one PLEASE help me!!!!! Thanks? | Socratic
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
# Vicarious-Nucleophilic-Substitution-Para-X-Chlorine-and-EWG-Nitrile
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
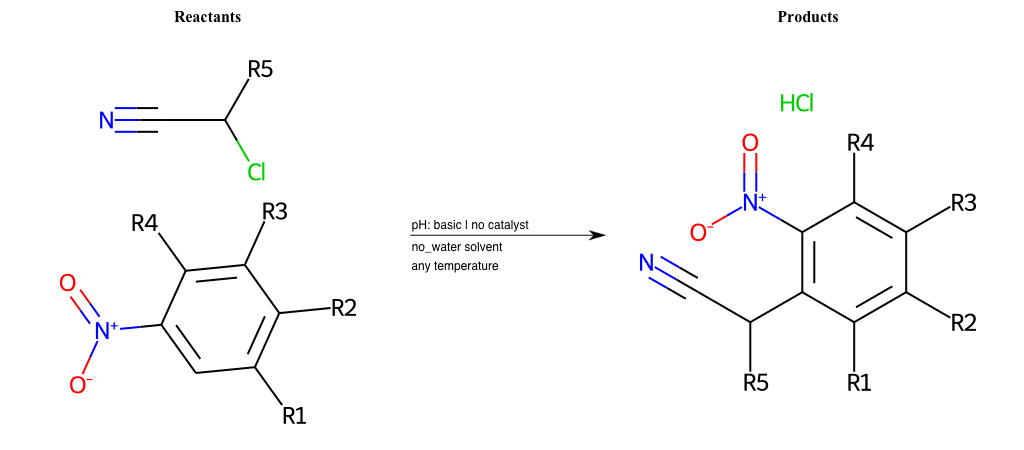
# Nucleophilic-Aromatic-Substitution-Lg-Fluorine-and-Nu-Hydroxyl
References:
[0]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[1]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[2]
Nucleophilic Aromatic Substitution - Chemistry Steps
[3]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
[4]
Explain why the trifluoromethyl (CF_3) group is meta directing in electrophilic aromatic substitution. What would you expect CF_3 to be activating or deactivating? Why? Can some one PLEASE help me!!!!! Thanks? | Socratic
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
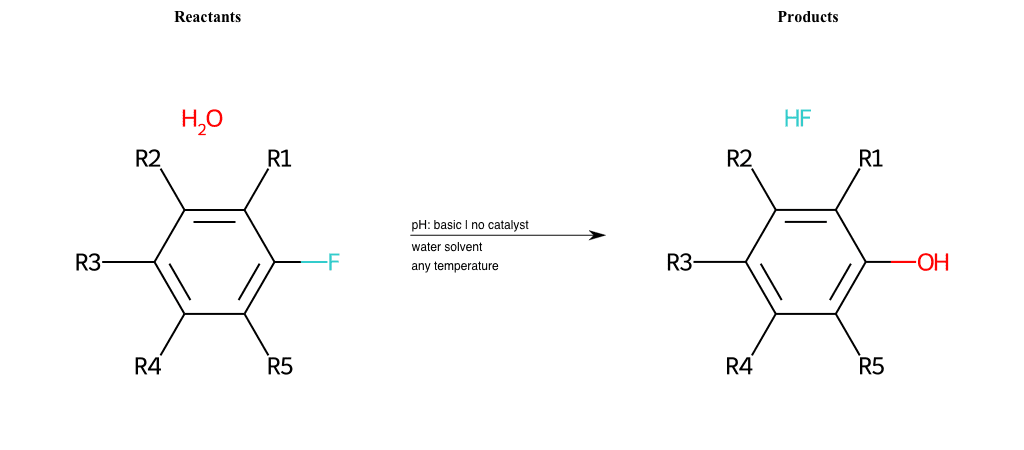
# Nucleophilic-Aromatic-Substitution-Pyridine-Ortho-Lg-Fluorine-and-Nu-Amino
References:
[0]
organic chemistry - Why does nucleophilic aromatic substitution occur at C-2 and C-4 of pyridine? - Chemistry Stack Exchange
[1]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[2]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[3]
Nucleophilic Aromatic Substitution - Chemistry Steps
[4]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
Condition to enforce:
R5 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
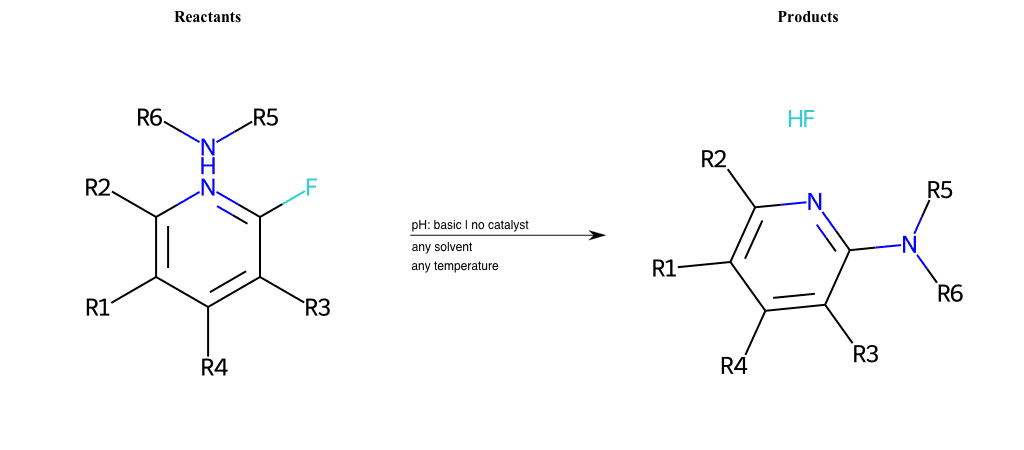
# Vicarious-Nucleophilic-Substitution-Ortho-X-Chlorine-and-EWG-Sulfonyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
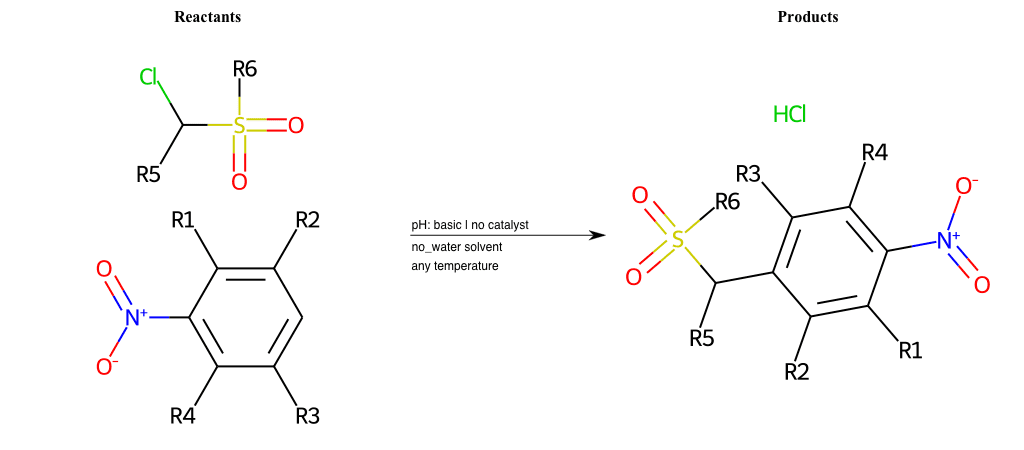
# Substituted-Pyridine-Nucleophilic-Aromatic-Substitution-Lg-Chlorine-and-Nu-Amino
References:
[0]
Weickgenannt_Jun_12.pdf
Condition to enforce:
R4 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
R5 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
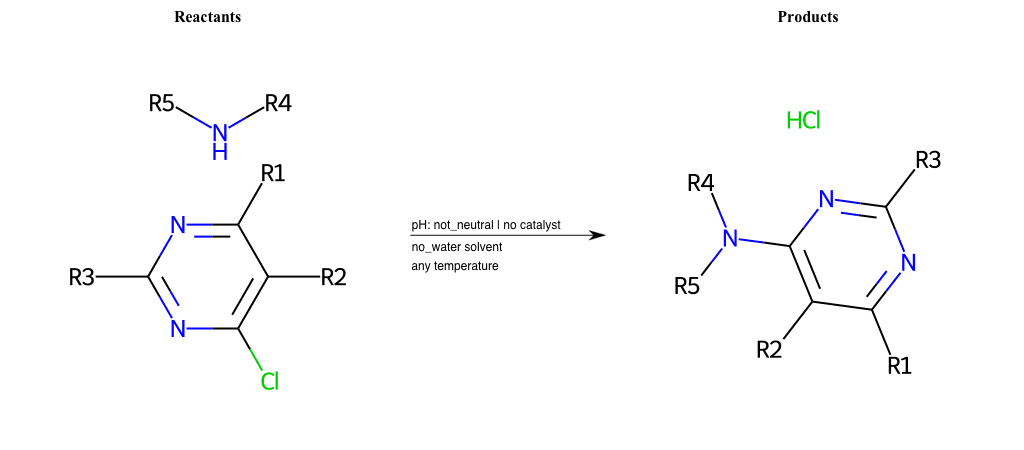
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Nitrite-and-EWG2-Phosphonate-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R8 = A-Aliphatic-Carbon, A-Aromatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
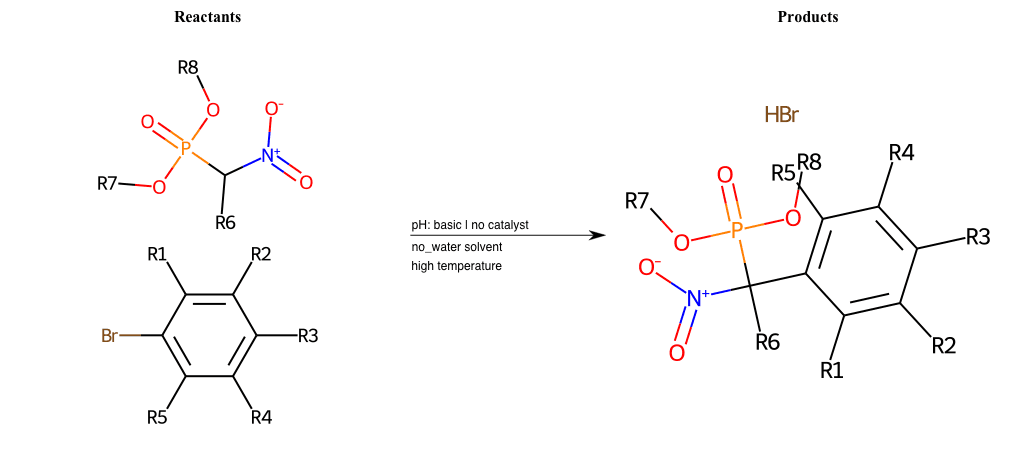
# Vicarious-Nucleophilic-Substitution-Ortho-X-Bromine-and-EWG-Carboxyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
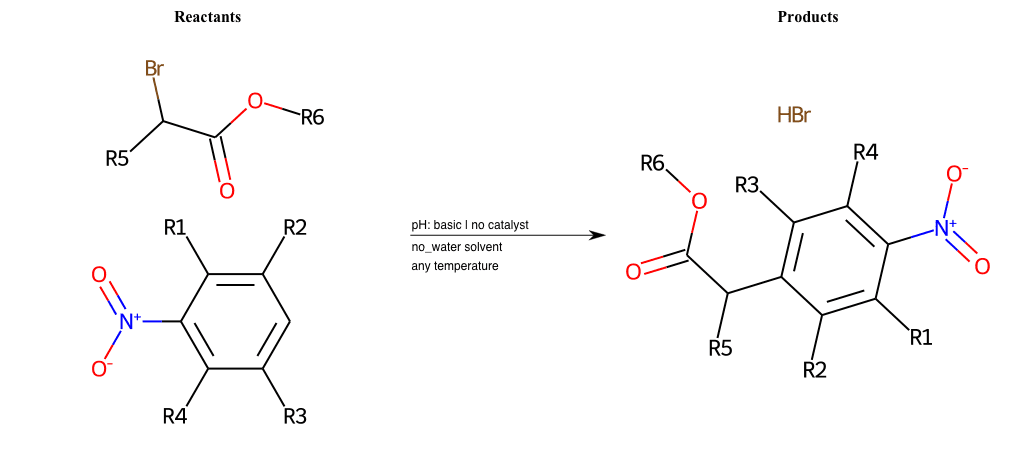
# Vicarious-Nucleophilic-Substitution-Para-X-Thiolate-and-EWG-Carbonyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R7 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
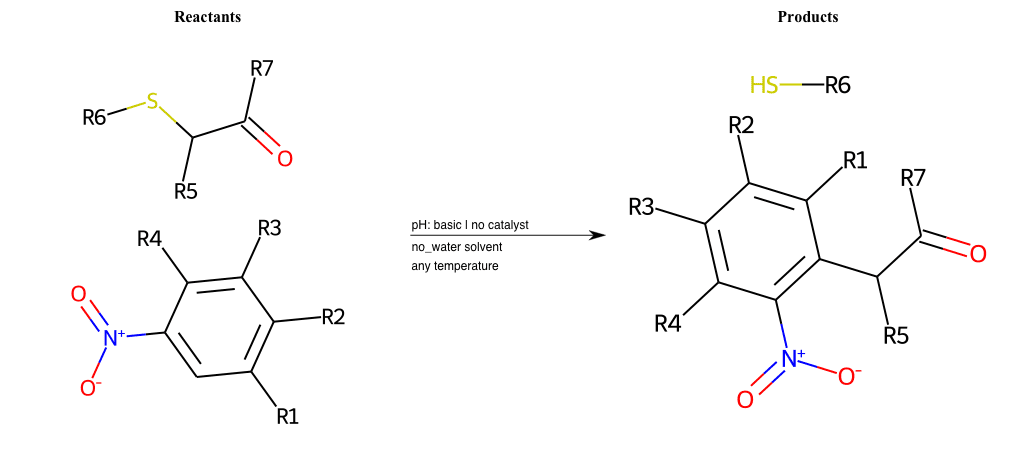
# Nucleophilic-Aromatic-Substitution-Pyridine-Ortho-Lg-Iodine-and-Nu-Hydroxyl
References:
[0]
organic chemistry - Why does nucleophilic aromatic substitution occur at C-2 and C-4 of pyridine? - Chemistry Stack Exchange
[1]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[2]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[3]
Nucleophilic Aromatic Substitution - Chemistry Steps
[4]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
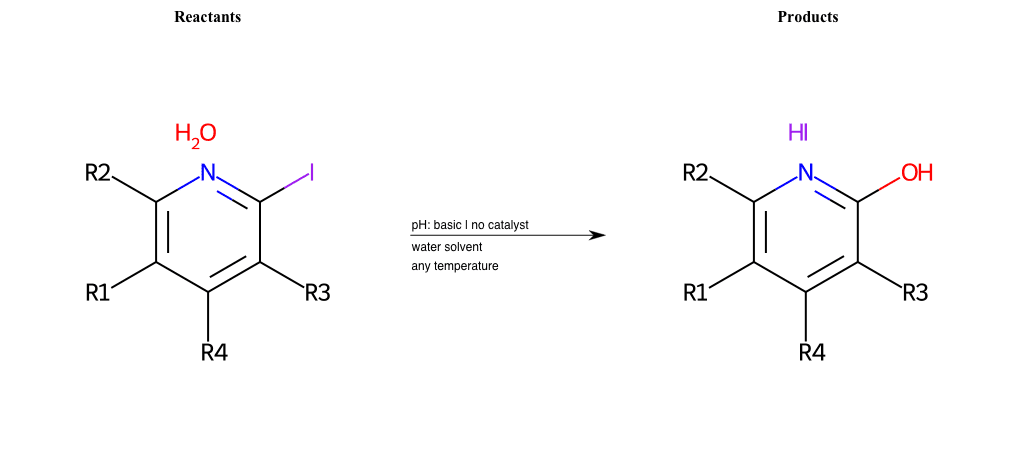
# Nucleophilic-Aromatic-Substitution-Pyridine-Ortho-Lg-Bromine-and-Nu-Hydroxyl
References:
[0]
organic chemistry - Why does nucleophilic aromatic substitution occur at C-2 and C-4 of pyridine? - Chemistry Stack Exchange
[1]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[2]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[3]
Nucleophilic Aromatic Substitution - Chemistry Steps
[4]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
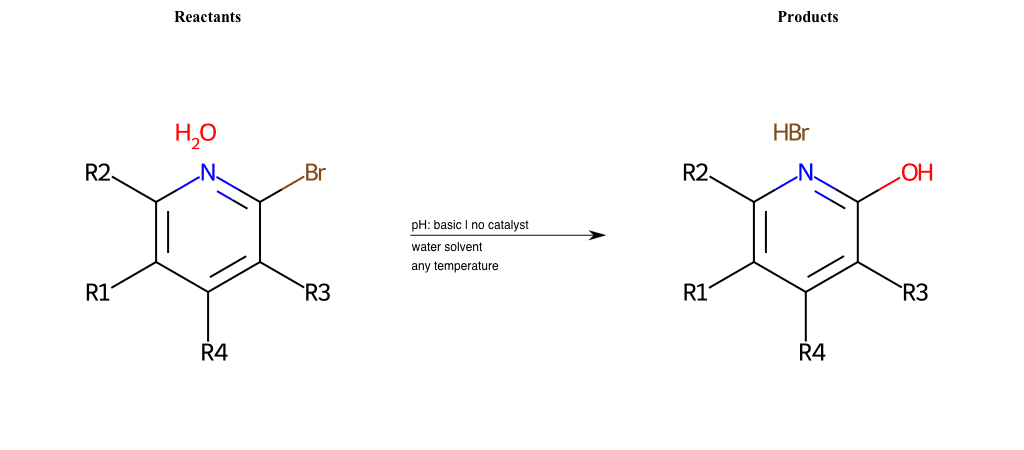
# Nucleophilic-Aromatic-Substitution-Pyridine-Para-Lg-Chlorine-and-Nu-Amino
References:
[0]
organic chemistry - Why does nucleophilic aromatic substitution occur at C-2 and C-4 of pyridine? - Chemistry Stack Exchange
[1]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[2]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[3]
Nucleophilic Aromatic Substitution - Chemistry Steps
[4]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
Condition to enforce:
R5 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
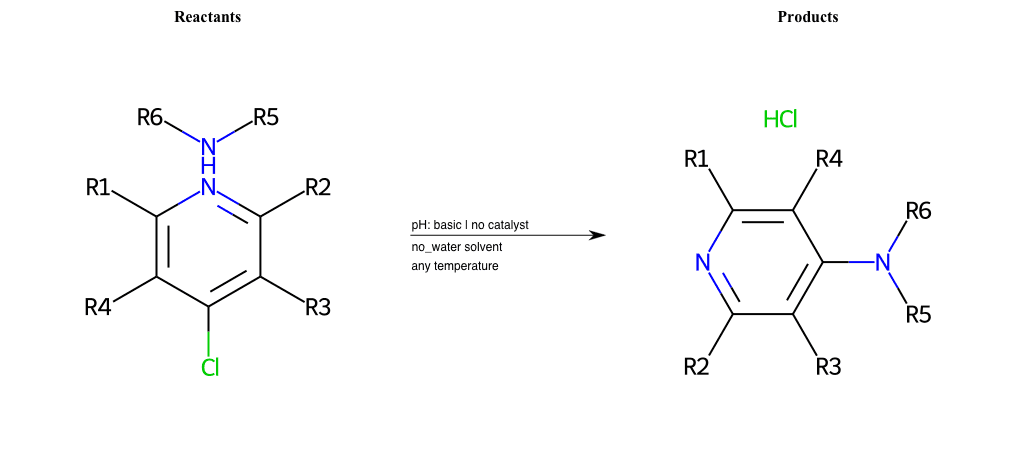
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carboxyl-and-EWG2-Carboxyl-Lg-Sulfonate
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
R8 = A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
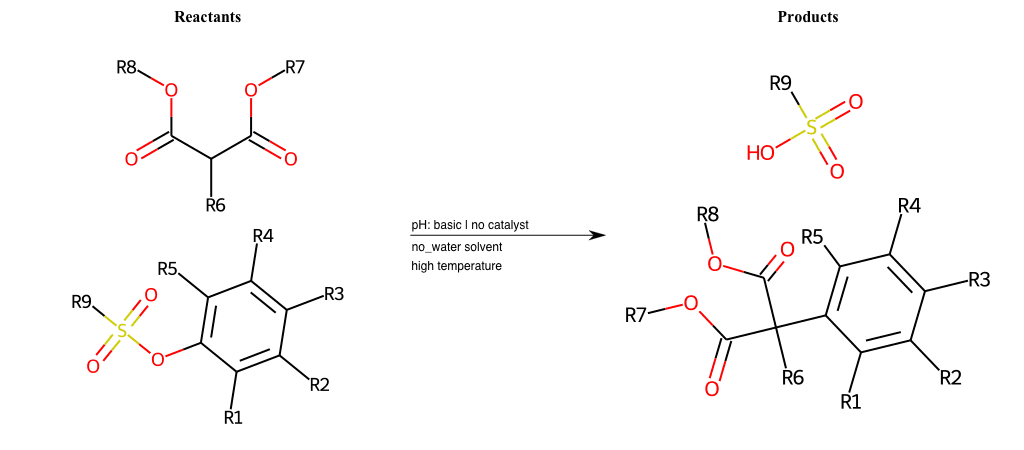
# Nucleophilic-Aromatic-Substitution-Lg-Chlorine-and-Nu-Alkoxide
References:
[0]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[1]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[2]
Nucleophilic Aromatic Substitution - Chemistry Steps
[3]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
[4]
Explain why the trifluoromethyl (CF_3) group is meta directing in electrophilic aromatic substitution. What would you expect CF_3 to be activating or deactivating? Why? Can some one PLEASE help me!!!!! Thanks? | Socratic
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = A-Aliphatic-Carbon, A-Aromatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
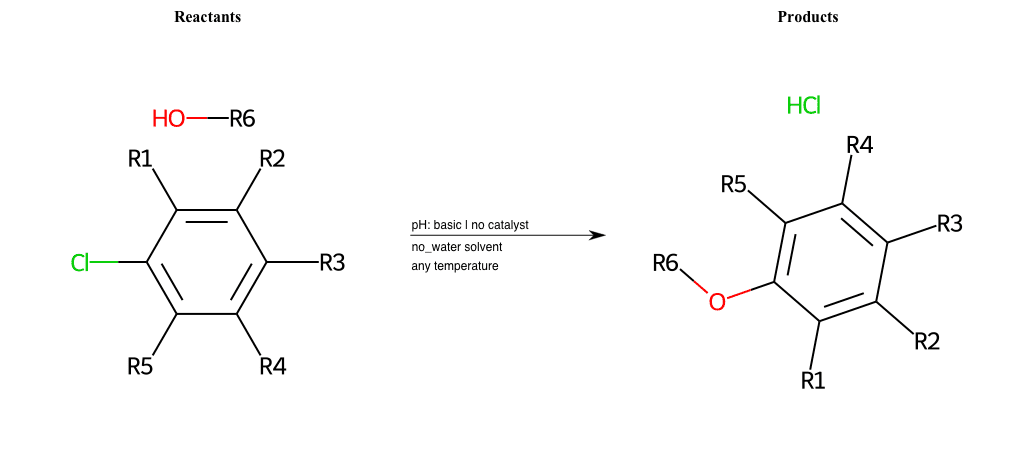
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Carbonyl-and-EWG2-Carbonyl-Lg-Bromine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
R8 = H, A-Aromatic-Carbon, A-Aliphatic-Carbon, Vinyl-Group
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
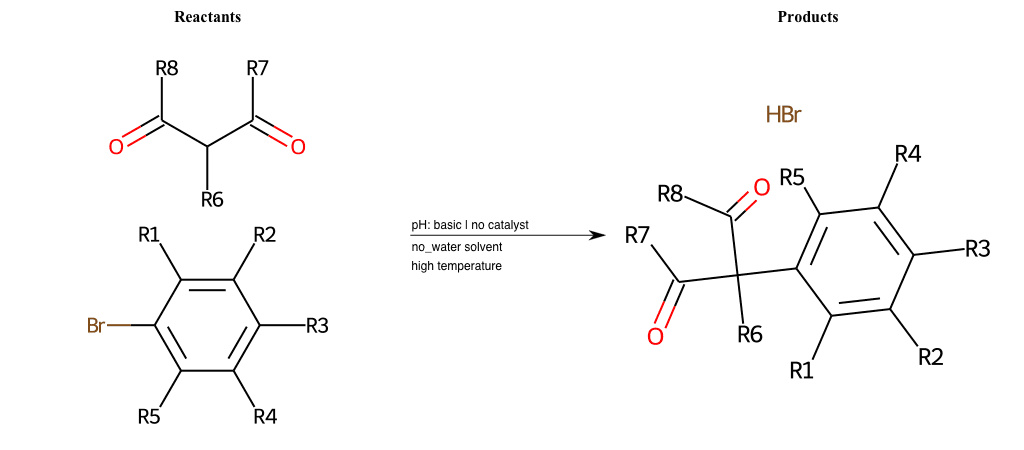
# Nucleophilic-Aromatic-Substitution-Nitric-Acid-Lg-Bromine
References:
[0]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[1]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[2]
Nucleophilic Aromatic Substitution - Chemistry Steps
[3]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
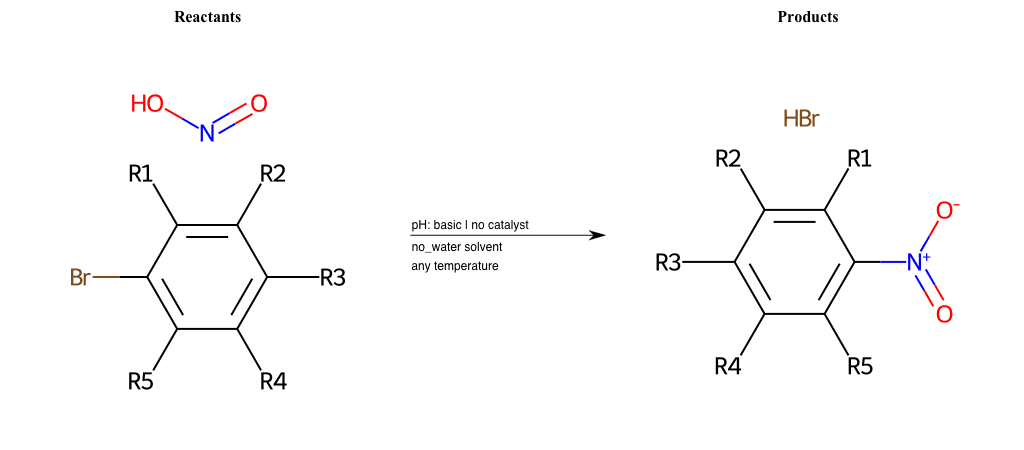
# Nucleophilic-Aromatic-Substitution-Lg-Bromine-and-Nu-Thiolate
References:
[0]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[1]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[2]
Nucleophilic Aromatic Substitution - Chemistry Steps
[3]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
[4]
Explain why the trifluoromethyl (CF_3) group is meta directing in electrophilic aromatic substitution. What would you expect CF_3 to be activating or deactivating? Why? Can some one PLEASE help me!!!!! Thanks? | Socratic
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
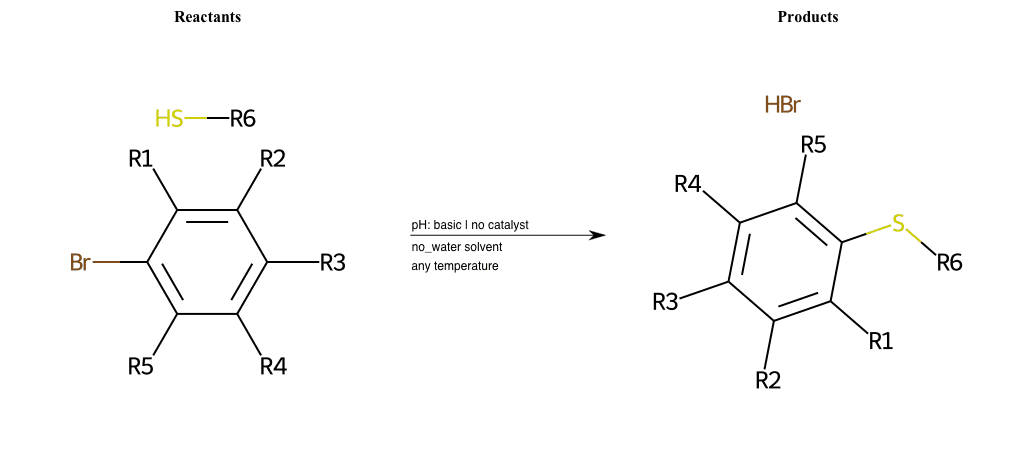
# Nucleophilic-Aromatic-Substitution-Alpha-Acid-EWG1-Nitrile-and-EWG2-Phosphonate-Lg-Chlorine
References:
[0]
Acetoacetic-Ester Synthesis
[1]
Substituted carbonyl compound synthesis by alkylation or condensation
[2]
Malonic Ester Synthesis
[3]
Carbonyl alpha-substitution reactions - Wikipedia
Condition to enforce:
R1 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R3 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R5 = L-A, A-Aliphatic-Carbon, H, Nitrite, Nitrile, Chlorine, Bromine, Iodine, Fluorine, Halocarbon
R6 = H, A-Aliphatic-Carbon
R7 = A-Aliphatic-Carbon, A-Aromatic-Carbon
R8 = A-Aliphatic-Carbon, A-Aromatic-Carbon
At least one logic:
Molecule conditions:
list_of_labels = R1, R3, R5
list_of_matching = (NO2L, Nitrite), (C#N[L], Nitrile), (ClL, Chlorine), (BrL, Bromine), (IL, Iodine), (FL, Fluorine), (CX3L, Halocarbon)
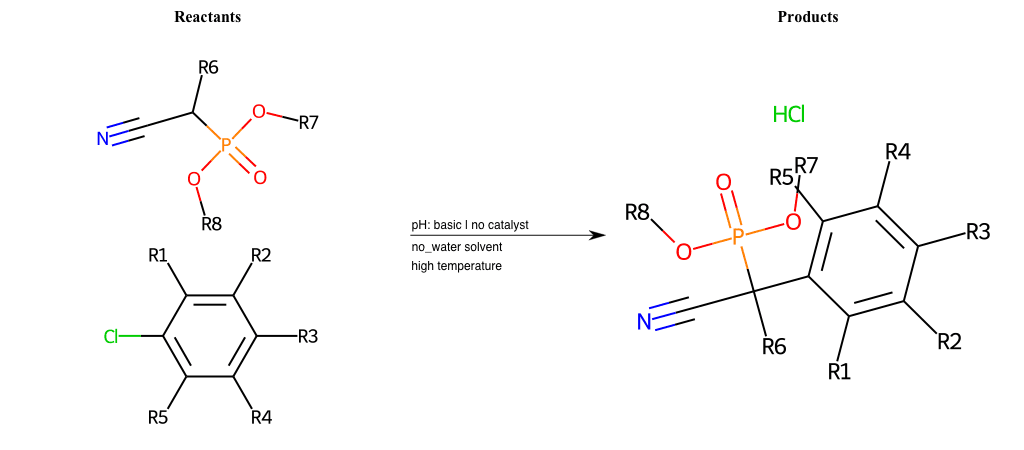
# Nucleophilic-Aromatic-Substitution-Pyridine-Ortho-Lg-Bromine-and-Nu-Amino
References:
[0]
organic chemistry - Why does nucleophilic aromatic substitution occur at C-2 and C-4 of pyridine? - Chemistry Stack Exchange
[1]
Nucleophilic Aromatic Substitution: Introduction and Mechanism
[2]
16.7: Nucleophilic Aromatic Substitution - Chemistry LibreTexts
[3]
Nucleophilic Aromatic Substitution - Chemistry Steps
[4]
Organic chemistry 29: Aromaticity - nucleophilic aromatic substitution, benzyne
Condition to enforce:
R5 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon, Vinyl-Group-No-Oxygen, A-Alkoxide, Hydroxyl, A-Aliphatic-Nitrogen
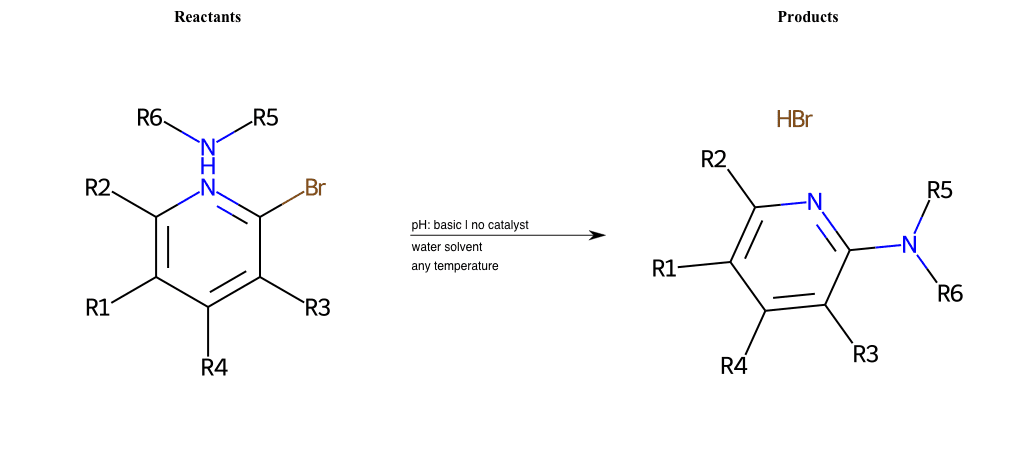
# Vicarious-Nucleophilic-Substitution-Ortho-X-Thiolate-and-EWG-Sulfonyl
References:
[0]
Vicarious nucleophilic substitution - Wikipedia
[1]
Just a moment…
Condition to enforce:
R1 = L-A
R2 = L-A
R3 = L-A
R4 = L-A
R5 = L-A
R6 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon
R7 = H, A-Aliphatic-Carbon, A-Aromatic-Carbon